Abstract
In this study, we report the analysis of 345 Klebsiella pneumoniae (K. pneumoniae) clinical isolates from patients hospitalized in various wards, of Perugia General Hospital, from February to December 2015. Among these isolates, 111 (32.2%) were resistant to at least one carbapenem antibiotic and all isolates were carbapenemase producers. The dissemination of Carbapenem- Resistant K. pneumoniae isolates (CRKPs) was not restricted to a high risk wards, but was observed in distinct hospital’s sectors. CRKPs were, mostly, isolated from respiratory tract and wound exudates. These results confirm the epidemic CRKPs dissemination, across our national territory, and the need for infection control and antibiotic stewardship programs.
Keywords: CRKP; K. pneumoniae; Carbapenemases
Abbreviations
CRKP: Carbapenem-Resistant Klebsiella Pneumoniae; K. pneumonaie: Klebsiella Pneumoniae
Introduction
Carbapenem-resistant K. pneumonaie isolates (CRKPs) cause serious infections, in debilitated and immunocompromised patients, with a high mortality rates, depending on the study population [1]. The respiratory tract is the most common site of infection, however urinary tract infection, catheter related and surgical site infections, commonly, also, occur [2].
Carbapenem-resistant K. pneumoniae isolates, have become, in the last years, a public health problem of major concern. Carbapenemase production, (KPC type), is the most important carbapenem resistance mechanism in these bacteria [3,4].
Since their discovery, 16 years ago [5], a rapid worldwide increase of carbapenemase producing K. pneumoniae has been documented [3,4]. This is, in part, due to the propensity of K. pneumoniae to acquire genetic material through horizontal gene transfer, to the ease of transmission of such microorganism among people (in particular through contaminated hands) and to the transfer of patients coming from hospitals where there had been outbreaks/epidemics caused by CRKPs. In Italy, these bacteria have been documented for the first time in late 2008 [6] and, since 2010, a rapid and extensive dissemination of carbapenem-resistant K. pneumoniae, across the national territory, has been reported [7-12].
Guidelines, from European Centre for Disease Prevention and Control, to prevent and control the spread of these bacteria, have been established [13]. In Italy, the Ministry of Health issued, in February 2013, a circular letter [14] asking, the Italian regions, to report all cases of bloodstream infections due to carbapenemase- producing K. pneumoniae or E.coli and recommending control measures to limit the spread in healthcare setting.
The aim of this study was to evaluate the incidence of K. pneumoniae isolates showing resistance to carbapenems, among patients hospitalized at the Perugia General Hospital, from February to December 2015, to set up interventions able to prevent and control the spread of these threatening microorganisms.
Materials and Methods
From February to December 2015, a total number of 345 nonreplicate clinical isolates of K. pneumoniae, recovered from patients hospitalized in Medical wards, Surgical wards, Intensive Care Units and Hematology/Oncology wards, were collected at the Clinical Microbiology Laboratory of the Perugia General Hospital, Italy. According to the guidelines of Clinical and Laboratory Standards Institute (CLSI) [15] and of European Antimicrobial Resistance Surveillance Network (EARS-Net) [16], we included, in this study, only the first isolate per patient, irrespective of the body site from which the specimen was obtained or the antimicrobial susceptibility pattern. Isolates from surveillance or screening cultures were excluded from the study.
The isolates were recovered from several clinical samples: urine, respiratory tract, blood, wound exudates or other biological material. Bacterial identification was carried out by Matrix-Assisted Laser Desorption Ionisation-Time of Flight Mass Spectrometry(MALDITOF MS) (Bruker Daltonics, Bremen, Germany), as previously described by Mencacci et al. [17]. Antimicrobial susceptibility testing was performed by using the Phoenix Automated Microbiology System (Becton Dickinson Diagnostic Systems, Sparks, United States) or the Vitek-2 System (bioMérieux, Marcy l’Etoile, France). Confirmatory MIC testing, for meropenem, imipenem and ertapenem, was performed by Etest (bioMérieux, France). Antimicrobial MICs were interpreted using clinical breakpoints, according to European Committee on Antimicrobial Susceptibility Testing (EUCAST): =2 μg/mL for meropenem-susceptibility, >8 μg/mL for meropenemresistance, =2 μg/mL for imipenem-susceptibility, >8 μg/mL for imipenem-resistance, =0.5 μg/mL for ertapenem-susceptibility and >1 μg/mL for ertapenem-resistance [18]. All isolates with meropenem MIC >0.125 μg/ml, Epidemiological Cut- Off Value (ECOFF) according to EUCAST guidelines [19], were tested for carbapenemases production, by means of commercial KPC+MBL Confirm ID kit (Rosco Diagnostica A/S, Denmark) [20], and results were interpreted according to the manufacturer’s instruction. The isolates non-susceptible to meropenem were considered as CRKP for the purpose of this study. Detection of blaKPC gene was performed by a commercial multiplex PCR (hyplex SuperBug ID), according to the manufacturer’s directions (Amplex Diagnostics GmbH, Germany).
Data analysis was performed by using of WHONET software, version 5.6, a software program for the management of microbiology laboratory data that is available, free of charge, from the World Health Organization [21].
Results and Discussion
During the study period (February-December 2015), a total of 345, non-replicate, clinical isolates of K. pneumoniae, were identified from patients admitted to the Perugia General Hospital. Among these isolates, 111 (32.2 %) were resistant to, at least, one carbapenem antibiotic (all were resistant to meropenem, 98.2% to imipenem and 99.1% to ertapenem). All the CRKPs produced carbapenemases and all harboured the blaKPC gene.
The hospital distribution of total K. pneumoniae and CRKP isolates is shown in Figure 1(A, B). Among the 111 isolates, 61.3% (n=68) were from Medical wards, 18% (n=20) from Intensive Care Units, 15.3% (n=17) from Surgical wards and 5.4% (n=6) from Hematology/ Oncology wards. The proportion of CRKPs, over the total number of K. pneumoniae strains isolated from the same hospital sector, was: 32.7% (n=68/208) in Medical wards, 32.2% (n=20/62) in Intensive Care Units, 31.6% (n= 6/19) in Hematology/Oncology wards and 30.3% (n=17/56) in Surgical wards. These results showed that the dissemination, of carbapenem-resistant K. pneumoniae isolates, was not limited to a high risk wards, but disseminated in multiple hospital sectors. Although frequent bed transfer of patients, particularly after isolation of CRKPs, combined with the lack of adequate preventive measures, might have facilitated this process, the propensity, of these bacteria, to spread in multiple wards should be considered when planning infection control strategies.
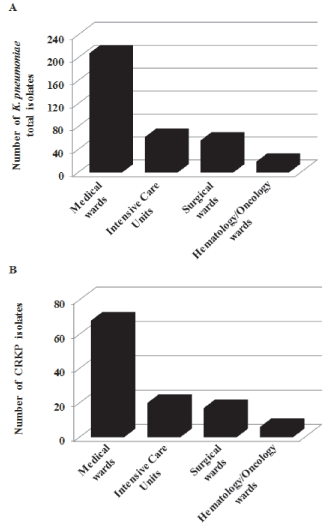
Figure 1: Distribution of K. pneumoniae total isolates (A) and carbapenemresistant
K. pneumoniae isolate (B) according to hospital wards.
The biological source of K. pneumoniae, total and CRKP, isolates was, also, evaluated. Among 345 total K. pneumoniae isolates, 124 were from urine, 73 from respiratory tract, 56 from wound exudates, 37 from blood, and 55 from other body sites. Regarding the 111 CRKPs, 32 were from urine, 23 from respiratory tract, 18 from wound exudates, 8 from blood and 30 from other anatomic sites. The higher proportion of CRKP isolates, over the total number of K. pneumoniae strains isolated from the same clinical specimens, was observed for wound exudates (32.1%; n=18/56) and respiratory tract (31.5%; n=23/73), followed by urine (25.8%; n=32/124) and blood (21.6%; n=8/37). The proportion of CRKPs isolates from other body sites was 54.5% (n=30/55).
In Table 1 the distribution of total K. pneumoniae and CRKP isolates, according to clinical samples and hospital wards, is shown. The higher percentage of CRKPs was observed in Surgical and Medical wards for respiratory tract, respectively, 75% (n=3/4) and 37.1% (n=13/35), in ICU for urine (42.8%; n=3/7) and in Hematology/ Oncology for urine (33.3%; n=1/3) and wound exudates (33.3%; n=1/3). It is probable that this clinical feature could be related to different devices used during the hospitalisation. Given that CRKPs have a remarkable potential for causing life threatening infections [22], it is important to note that CRKPs were not isolated from blood in patients hospitalized in Intensive Care Units, in Hematology/ Oncology and Surgical wards.
Wards
Isolates
Urine
Respiratory tract
Wound exudates
Blood
Total
CRKP (%)
Total
CRKP (%)
Total
CRKP (%)
Total
CRKP (%)
Medical wards
94
22 (23.4)
35
13 (37.1)
31
11 (35.5)
24
8 (33.3)
Surgical wards
20
6 (30)
4
3 (75)
17
5 (29.4)
5
0
Intensive Care Units
7
3 (42.8)
29
6 (20.7)
5
1 (20)
7
0
Hematology/Oncology wards
3
1 (33.3)
5
1 (20)
3
1 (33.3)
1
0
Table 1: Distribution of total K. pneumoniae isolates and carbapenem-resistant K. pneumoniae isolates (CRKPs) according to clinical samples and Perugia General Hospital wards.
Conclusion
Infections caused by CRKPs have limited treatment options and have been associated with high mortality rates [22,23]. In Italy, to date, CRKPs have reached epidemic dissemination. Our study show the presence of a significant percentage of CRKP isolates in Perugia General Hospital with a dissemination in multiple wards. These results confirm the need for infection control, antibiotic stewardship programs and the utilization of a surveillance and prevention system, in order to reduce the selective pressure that favors the emergence and the consequent spread of carbapenem-resistant isolates.
Acknowledgement
This work was supported by “Fondazione Cassa di Risparmio” of Perugia, Italy (grant No. 2014.0221.021).
References
- Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect. 2011; 17: 1798-1803.
- Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013; 34: 1-14.
- Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011; 17: 1791-1798.
- Cantón R, Akóva M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect. 2012; 18: 413-431.
- Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001; 45: 1151-1161.
- Giani T, D’Andrea MM, Pecile P, Borgianni L, Nicoletti P, Tonelli F, et al. Emergence in Italy of Klebsiella pneumoniae sequence type 258 producing KPC-3 Carbapenemase. J Clin Microbiol. 2009; 47: 3793-3794.
- Gaibani P, Ambretti S, Berlingeri A, Gelsomino F, Bielli A, Landini MP, et al. Rapid increase of carbapenemase-producing Klebsiella pneumoniae strains in a large Italian hospital: surveillance period 1 March - 30 September 2010. Euro Surveill. 2011; 16: 1-3.
- Giani T, Pini B, Arena F, Conte V, Bracco S, Migliavacca R. AMCLI-CRE Survey Participants, Pantosti A. Epidemic diffusion of KPC carbapenemaseproducing Klebsiella pneumoniae in Italy: results of the first countrywide survey, 15 May to 30 June 2011. Euro Surveill. 2013; 18.
- Fontana C, Favaro M, Sarmati L, Natoli S, Altieri A, Bossa MC, et al. Emergence of KPC-producing Klebsiella pneumoniae in Italy. BMC Res Notes. 2010; 3: 40.
- Ambretti S, Gaibani P, Caroli F, Miragliotta L, Sambri V. A carbapenemresistant Klebsiella pneumoniae isolate harboring KPC-1 from Italy. New Microbiol. 2010; 33: 281-282.
- Agodi A, Voulgari E, Barchitta M, Politi L, Koumaki V, Spanakis N, et al. Containment of an outbreak of KPC-3-producing Klebsiella pneumoniae in Italy. J Clin Microbiol. 2011; 49: 3986-3989.
- Monari C, Merlini L, Nardelli E, Cacioni M, Repetto A, Mencacci A, et al. Carbapenem-Resistant Klebsiella pneumoniae: Results of a Laboratory Surveillance Program in an Italian General Hospital (August 2014-January 2015). Adv Exp Med Biol. 2016; 26.
- European Centre for Disease Prevention Control (ECDC) (2013) Antimicrobial resistance surveillance in Europe 2012. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net).
- Ministero della Salute. Sorveglianza e controllo delle infezioni da batteri produttori di carbapenemasi (CPE). DGPRE. 2013.
- CLSI. Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data; Approved Guideline-Fourth Edition. CLSI document M39-A4. Wayne, PA: Clinical and Laboratory Standards Institute. 2014.
- European Centre for Disease Prevention Control. Antimicrobial resistance surveillance in Europe 2012. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: ECDC. 2013.
- Mencacci A, Monari C, Leli C, Merlini L, De Carolis E, Vella A, et al. Typing of nosocomial outbreaks of Acinetobacter baumannii by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2013; 51: 603-606.
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 5.0. 2015.
- European Committee on Antimicrobial Susceptibility. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/ or epidemiological importance. 2013.
- Giske CG, Gezelius L, Samuelsen Ø, Warner M, Sundsfjord A, Woodford N. A sensitive and specific phenotypic assay for detection of metallo-β- lactamases and KPC in Klebsiella pneumoniae with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin. Clin Microbiol Infect. 2011; 17: 552-556.
- World, Health, Organization. WHONET software.
- Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N, Nativ R, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol. 2009; 30: 972-976.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008; 29: 1099-1106.
