Abstract
Pleomorphic Adenoma (PA) is an epithelial tumor of complex morphology, comprising of epithelial and myoepithelial elements arranged in a variety of patterns and surrounded by a mucopolysaccharide stroma, whereas Carcinoma ex Pleomorphic Adenoma (CXPA) is characterized by the presence of malignant transformation in pleomorphic adenoma and it is one of the rare, aggressive and poorly understood malignancies of the salivary gland. Our study is to emphasize that the histopathology is the gold standard for making the diagnosis of CXPA.
Keywords: Pleomorphic adenoma; CXPA; Mucoepidermoid carcinoma; Histopathology; Loss of heterozygosity
Introduction
Carcinoma ex pleomorphic adenoma is defined as a carcinoma arising from epithelial or myoepithelial or both the components of a primary or recurrent benign PA [1]. Malignant changes in PA have been associated with long duration, tumor size, tumor recurrence, radiation therapy and advanced age [2]. In majority of the cases (75%), luminal epithelial cells undergo malignant transformation. CXPA more commonly occurs in the major salivary glands than in the minor salivary glands. Further in major salivary glands, parotid (67%) is the most frequently affected gland and sublingual is the least affected one (<1%).CXPA has the highest false-negative rate of 35.3% of all malignant salivary gland tumour, which is due to its tendency to involve the deep lobe, in contrast to the Pleomorphic adenoma which tends to involve the superficial lobe [3]. This is one of the reasons for delay in diagnosis through Fine Needle Aspiration Cytology. And being a rare carcinoma with considerable false-negativity, it often poses a great challenge to clinicians as well pathologists.
Case Presentation
A 50-year-old male presented with a painless mass in the right preauricular area since 5 years, for which he underwent FNAC and report showed Pleomorphic Adenoma. But, the patient did not take any treatment. A sudden rapid growth was noticed in the last 3 months, for which patient consulted the surgeon. On extra-oral examination, the mass was ovoid in shape, firm in consistency, mobile, non-tender and measuring about 4x3 cm in size. The skin over the swelling was normal. CT and MRI showed a single ovoid well-circumscribed mass in the right parotid space measuring about 3x2x1.5 cm (Figure 1). Then the patient was advised excisional biopsy of the lesion. Macroscopically, the mass was ovoid, firm with nodular surface and completely encapsulated. Cut surface of the mass was greyish-white in colour (Figure 2). Microscopically, the soft tissue section showed an encapsulated tumor composed of a mixture of ductal epithelial and myoepithelial cells in a hyalinized mesenchymal background with the absence of infiltration of tumor cells in the peripheral serous acini and adipose cells (Figure 3). Foci of section showed neoplastic epithelial cells which were cuboidal in shape and arranged in the form of sheets and ducts filled with eosinophilic material and neoplastic myoepithelial cells are mostly plasmacytoid type, which were arranged in sheets and interlacing cords (Figure 4). Another part of the section showed capsular infiltration of sheets of neoplastic cells. Some areas showed MEC pattern with Sheets of epidermoid cells and mucoid cells. Plasmacytoid cells showed nuclear pleomorphisim with open faced nucleus and many nucleoli. Another part of the section shows squamous metaplasia of neoplastic cells (Figure 5 & 6). Immunohistochemistry - myoepithelial cells showed positivity for S100 and SMA (Figure 7). All the above features were pointing towards a carcinoma in addition to Pleomorphic Adenoma. Thereby a diagnosis of minimally invasive CXPA with the malignant component of mucoepidermoid carcinoma was made. Lobectomy of the right parotid was done. No recurrence and Metastasis was noticed till now.
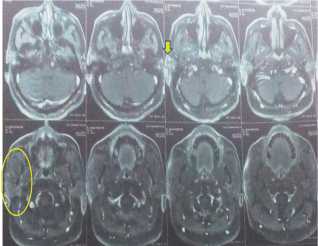
Figure 1: MRI showed a single ovoid well-circumscribed mass in the right
parotid space.
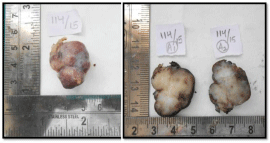
Figure 2: Grossing specimen shows ovoid, firm, nodular surface.
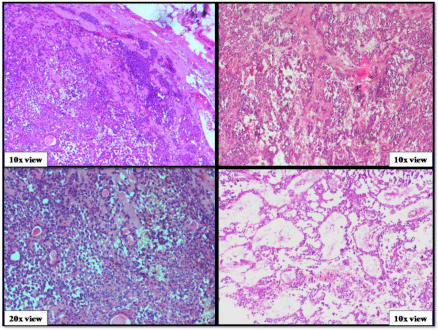
Figure 3: Benign component of pleomorphic adenoma.
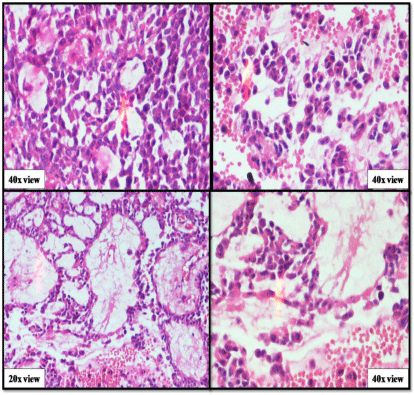
Figure 4: Epithelial and myoepithelial cells of pleomorphic adenoma.
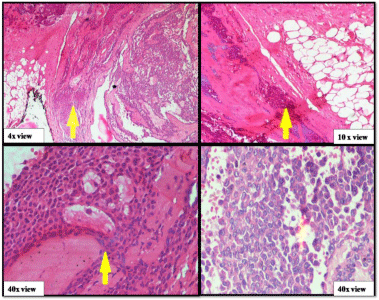
Figure 5: Malignant component of pleomorphic adenoma (capsular
infiltration, MEC pattern, cellular pleomorphism).
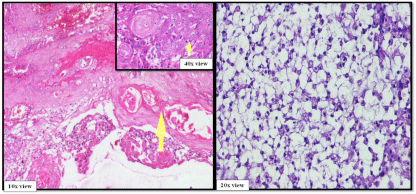
Figure 6: Malignant component of pleomorphic adenoma (Squamous
metaplasia, MEC pattern).
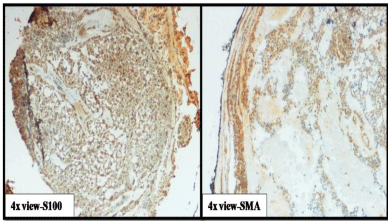
Figure 7: Immunohistochemistry-positivity of myoepithelial cells to S100 &
SMA.
Discussion
CXPA is the third common malignancy of the salivary glands and it comprises 3.6% of total salivary gland neoplasms, and 6.2% of all mixed tumours [4]. Commonly seen in 60 to 80 year age group with slight predilection towards females. According to WHO histological classification (2005), the “malignant mixed tumors,” should be divided into 3 different clinical and histological entities: 1) carcinoma ex pleomorphic adenoma, 2) Carcinosarcoma, and 3) metastasizing pleomorphic adenoma [2]. Malignant mixed tumors represent approximately 11.6% of all malignant neoplasms in the salivary glands. The term ‘Malignant mixed tumors’ is used in synonymous to CXPA, as the majority of them are composed of CXPA only [5].
Clinical features
Typical presentation of CXPA is a longstanding history (average, 10 -15 years) of PA followed by a sudden rapid growth (average, 3- 6 months). Pain and facial nerve paralysis are often present [6]. This was the only case which was reported in the parotid gland in our institution between 2012-2016 (Table 1).
S.NO.
AGE OF THE PATIENT
SEX
SITE
C/O OF THE PATIENT
DURATION OF THE LESION
DIAGNOSIS
FOLLOW UP (ONE YEAR)
1
60 Years
Female
Parotid gland
Pain&Swelling
8months
Epithelial-Myoepithelial carcinoma
No
Recurrence
2
50 Years
Male
Hard palate
Pain&Swelling
6 Months
Carcinoma Ex Pleomorphic adenoma
Yes
3
35 Years
Male
Left side of Lower lip
Swelling
7 Months
Mucoepidermoid carcinoma
Not available
4
11 Years
Male
Left Buccal Mucosa
Swelling
2 Months
Mucoepidermoid Carcinoma
No Recurrence
5
27 Years
Female
Right Buccal Mucosa
Swelling
3 months
Mucoepidermoid carcinoma
Not available
6
43 Years
Male
Floor of Mouth
Pain & Swelling
1 Month
Mucoepidermoid carcinoma
Yes
7
56 Years
Female
Left Side of Lower lip
Pain & Swelling
2 Months
Adenoid Cystic Carcinoma
No
8
26 Years
Male
Right side of Lower lip
Swelling
2 Months
Intraductal papilloma of salivary gland
No
9
49 Years
Female
Retro molar area
Pain & Swelling
1 Month
Polymorphous Low grade Adenocarcinoma
Yes
10
52 Years
Male
Floor of the mouth
Pain & Swelling
4 Months
Salivary Duct Carcinoma
No
11
42 Years
Female
Hard Palate
Pain & Swelling
3 Months
Salivary Duct Carcinoma
Not available
12
37 Years
Female
Right Buccal Mucosa
Pain
3 Months
Adenoid Cystic Carcinoma
No
13
18 Years
Male
Left lower lip
Swelling
7 months
Adenoid Cystic Carcinoma
No
14
51 Years
Female
Buccal mucosa
Pain& Swelling
5 Months
Carcinoma Ex Pleomorphic adenoma
Yes
15
72 Years
Male
Hard palate
Swelling
3 months
Polymorphous Low grade Adenocarcinoma
No
16
50 Years
Male
Parotid gland
swelling
5 Years
Carcinoma Ex Pleomorphic adenoma
No
Table 1: Clinico pathologic findings of salivary gland tumors reported from 2012-2016.
We got in our case the patient was almost asymptomatic except for mild swelling with a sudden rapid growth. CXPA mostly affects major salivary glands (80%), among which parotid and submandibular are mostly affected. In case of minor salivary glands (20%) palate is commonly affected. Pain may be due to penetration of tumor into surrounding tissues [7]. Other symptoms include facial nerve palsy, skin ulceration and fixation, lymphadenopathy, and dysphasia [8,9].
The ratio of adenomatous and carcinomatous components determines the macroscopic features of CXPA. The tumour appears greyish-blue and transparent to yellowish-white, when the PA component is dominant, due to hyalinization and calcification of the stroma. If the malignant component is dominant, tumour appears widely infiltrative leading to necrosis and haemorrhage [1].
Based on morphological and Immunohistochemical features, CXPAs were classified into Carcinomas with Luminal Differentiation (CXPA-LD) and Non-Luminal Differentiation (CXPA-NLD) [10]. Based on the presence and extent of capsular invasion, CXPAs are subdivided into non-invasive, minimally invasive and invasive varieties. If there is no capsular infiltration of malignant cells it is non-invasive, if the invasion beyond the capsule is 1.5mm or lower it is minimally invasive and if the invasion is >1.5 mm it is invasive [4,11]. In our case capsular infiltration of malignant cells is <1.5 mm, so it is minimally invasive. In most cases (75%), the luminal epithelial cells undergo malignant change. In 19% of cases dual differentiation and in the remaining (6%) cases myoepithelial cell differentiation [6].
The most common malignant component is a poorly differentiated adenocarcinoma (NOS) or salivary duct carcinoma. Other malignant elements include mucoepidermoid, polymorphous low-grade adenocarcinoma, adenoid cystic carcinoma, clear cell or myoepithelial carcinoma [11]. In our case malignant component is mucoepidermoid carcinoma. Microsatellite analysis of CXPA has revealed that Loss of Heterozygosity (LOH) is common on chromosome 8q and 12q in both benign and malignant PA, suggesting the presence of tumor suppressor genes at these locations. LOH at chromosome 17p was reported in CXPA only [1,4]. LOH at chromosome 8q indicates initial steps in the pathogenesis of PA and LOH at chromosome 12q indicates adenomas with malignant potential, whereas LOH at 17p is indicative of terminal events in the carcinogenesis. Till now, the genes known to be involved in the malignant transformation are 12q, HMGIC, HMGA2 and MDM2 [1].
Diagnosis
Several diagnostic methods like Histopathology, Fine Needle Aspiration Cytology (FNAC), sonography, Computed Tomography (CT) scan, and Magnetic Resonance Imaging (MRI) scan are used in making the diagnosis. But pathological assessment is the gold standard among them. FNAC is commonly used pre-operatively to diagnose CXPA. Though CXPA may be seen by different CT appearances, it is difficult to diagnose by CT alone. Various CT appearances are: (a) it may be similar to a pleomorphism adenoma with no evidence of malignancy, (b) it may occur with focal necrosis, wall irregularity, or infiltrating margins, or (c) it may have an entirely aggressive CT appearance [12]. Preoperative radiologic diagnosis by conventional MRI also remains a challenge for radiologists, when it is presented with complicated components. As none of these preoperative diagnostic methods are accurate enough to diagnose when used alone, a combination of them is usually advised like in our case.
Carcinomas may rarely arise in a histologically benign adenoma (monomorphic adenoma) and must be differentiated from CXPA because of their good prognosis. The most important differential diagnosis is between non-invasive and minimally invasive CXPA, and the more typical invasive CXPA, since it has the prognostic significance and affects the decisions regarding need for lymph node dissection and adjuvant radiotherapy [13].
Treatment for CXPA often involves an ablative surgical procedure which may or may not be followed by reconstructive surgery. Superficial parotidectomy is indicated for intracapsular or minimally invasive CXPA whereas total or radical parotidectomy is for invasive variety and care should be taken to secure the facial nerve. A concomitant neck dissection is indicated for lymph nodal metastasis of CXPA. Post-operative radiotherapy is indicated in high grade cases [14].
Prognosis of CXPA depends on pathological staging factors like the level of invasion, lymph node involvement, and local or distant metastasis. According to LiVolsi and Perzin [15] the extent of tumor infiltration beyond the capsule is the most reliable prognostic marker. In patients with low grade tumors, the survival rate is 90% to 100%; Survival is better with tumors occurring in younger patients and among females, whereas survival rate is worse in patients older than 60 years of age [11,16]. Intermediate and high grade tumors have a greater tendency to invade, recur, and metastasize, with reported disease free rates at 5, 10, and15 years of 49%, 42%, and 33%, respectively [4].
Conclusion
CXPA is a rare complication of pleomorphic adenoma of de novo or recurrent origin in the parotid gland. It is known to occur in the elderly. A multi-staged pathogenesis is involved in development of CXPA. Though there are several modes of diagnostic methods, histopathology still plays a crucial role in accurate diagnosis as well in prognosis.
References
- Antony J, Gopalan V, Smith RA, Lam AKY. Carcinoma ex Pleomorphic Adenoma: A Comprehensive Review of Clinical, Pathological and Molecular Data. 2012; 6: 1-9.
- Kato H, KanematsuM, Mizuta K, Ito Y, Hirose Y. Carcinoma Ex Pleomorphic Adenoma of the Parotid Gland: Radiologic-Pathologic Correlation with MR Imaging Including Diffusion-Weighted Imaging. AJNR Am J Neuroradiol. 2008; 29: 865-867.
- VaniPadmaja GJ, Sireesha A, Devi T S, Nirmala BV. Carcinoma ex Pleomorphic Adenoma (CXPA)-A rare parotid malignancy. Indian Journal of Mednodent and Allied Sciences. 2013; 1: 54-58.
- Douglas R Gnepp. Diagnostic surgical pathology of the head and neck - 2nd ed. ISBN-13: 978-1-4160-2589-4.
- Akan H, Yildiz L, Unal R. Carcinoma ex pleomorphic adenoma of the minor salivary gland with pulmonary metastasis. Diagn Interv Radiol. 2008; 14: 3-5.
- Sandhya T, Avinash T, Treville P, Naik S. Oral Hyg Health: Carcinoma Ex Pleomorphic Adenoma: Rare Malignant Salivary Gland Neoplasm. 2014.
- Zbären P, Zbären S, Caversaccio MD, Stauffer E. Carcinoma ex pleomorphic adenoma: diagnostic difficulty and outcome. Otolaryngol Head Neck Surg. 2008; 138: 601-605.
- Nouraei SA, Hope KL, Kelly CG, McLean NR, Soames JV. Carcinoma ex benign pleomorphic adenoma of the parotid gland. Plast Reconstr Surg. 2005; 116: 1206-1213.
- Olsen KD, Lewis JE. Carcinoma ex pleomorphic adenoma: a clinicopathologic review. Head Neck. 2001; 23: 705-712.
- Kim JW, Kwon GY, Jong-Lyel Roh, Seung-Ho Choi, Nam SY, Kim SY, et al. Carcinoma ex Pleomorphic Adenoma of the salivary Glands: Distinct Clinicopathologic Features and Immunoprofiles between Subgroups According to Cellular Differentiation. J Korean Med Sci. 2011; 26: 1277-1285.
- Leon Barnes. Volume 1, Surgical Pathology of the head and neck Third Edition. University of Pittsburgh.
- Som PM, Brandwein MS. Salivary glands. Som PM, Curtin HD, editors. In: Head and neck imaging. St Louis: Mosby. 2003; 2070.
- Nagler RM, Laufer D. Synchronous pleomorphic adenomas of the major salivary glands: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 87: 735-737.
- Stodulski D, Rzepko R, Kowalska B, Stankiewicz C. Carcinoma ex pleomorphic adenoma of major salivary glands--a clinicopathologic review. Otolaryngol Pol. 2007; 61: 687-693.
- Mag SA, Cotulbea1 AH, Marin1 C, Doros D, Neamtu1 N, Balica1 A, et al. Carcinoma ex pleomorphic adenoma in parotid gland - case report. Journal of Experimental Medical & Surgical Research Cercetãri Experimentale & Medico-Chirurgicale Year XVII. 2010; 205-209.
- El-Naggar AK, Callender D, Coombes MM, Hurr K, Luna MA, Batsakis JG. Batsakis Molecular Genetic Alterations in Carcinoma Ex-Pleomorphic Adenoma: A Putative Progression Model? Genes, Chromosomes & Cancer. 200; 27: 162-168.
