Abstract
Sudden infants’ death is one of the most important matters in forensic medicine. The ability to pose a differential diagnosis between internal/infection and external/violent death is of paramount importance. Here, we report a case of a sudden death of an infant due to early-onset Group B Streptococcal (GBS) sepsis diagnosed by post-mortem microbiology analysis, since the mother was negative at vaginorectal GBS screening by culture. This case report highlights the importance of rapid and accurate nucleic acid amplification tests to detect GBS carriage status, especially in the delivery room.
Keywords: Post-mortem microbiology; Sudden infant death; Group B streptococci
Introduction
Streptococcus agalactiae or Group B Streptococcus (GBS) is a beta-hemolytic, catalase negative aerobe/anaerobe-facultative organism [1], a common commensal of the gastrointestinal and/or genitourinary tract in 10-30% of pregnant women [1,2]. GBS is also capable of causing severe infections, such as neonatal bacteremia, pneumonia, and meningitis [3], and severe invasive infections both in pregnant women and in non-pregnant adults associated with significant mortality [1,4]. Neonatal GBS infections may present as either fulminating septicemia or with subtle and non-specific early signs that overlap with those of non-infectious diseases. If not promptly treated with targeted antibiotic therapy, GBS infection may lead to rapid clinical deterioration represented by septic shock, multiorgan failure and disseminated intravascular coagulopathy [5].
The transmission of Group B Streptococcus between mother and her newborn is considered an important risk factor that could significantly increase the probability of the development of GBS disease [6,7]. Invasive neonatal GBS infections have been categorized in two different diseases, following the definition by CDC (https:// www.cdc.gov/groupbstrep/about/newborns-pregnant.html), namely Early Onset Disease (EOD) and Late Onset Disease (LOD). EOD is usually related to vaginal colonization of the mother and consequent vertical transmission during the delivery; it generally appears within 24 hours and occurs within the first week of life [8,9]. LOD occurs after the first week of life and within the first three months of life and it is usually secondary to horizontal transmission coming from nosocomial sources, such as the mother or other neonates [10,11]. EOD and LOD - besides their differences in their clinical presentation, mortality, and morbidity - also differ in epidemiological characteristics and proportion of GBS serotypes causing invasive infection [5]. They are generally associated with specific serotypes, mostly of serotypes III, Ia, V, Ib and II, accounting for approximately 95% of invasive disease (in decreasing frequency) [12], and clones, the most virulent being those belonging to clonal complexes CC17 and CC19, as defined by multi locus sequence typing [13].
Appropriate prenatal screening and administration of Intrapartum Antibiotic Prophylaxis (IAP) to mothers at risk of delivering GBS-infected infants has been found to reduce neonatal morbidity and mortality associated with EOD, while no effects have been reported on LOD [14,15]. Since the early 1990s, when IAP was implemented, the incidence of EOD has declined by approximately 80% [16], and EOD currently has slightly lower incidence rates than LOD [5]. Moreover, geographical variation in EOD incidence has been reported [17].
Sudden infant/neonate death is an important field in forensic microbiology [18]. Here, we report a case of a sudden death of an infant due to early-onset GBS sepsis with a negative vaginorectal at the mother, diagnosed by post-mortem microbiology analysis.
Case Presentation
A term female newborn (39 weeks, 3.050g weight) was born by vaginal birth by a healthy 25-year-old mother. No problems occurred during the delivery. Apgar scores were 10 at 1, 5 and 10 minutes after the birth. Vaginal and rectal swab cultures of the mother for prenatal screening, performed at the 35th and 37th week and prior to birth, were negative for BGS. Three days after birth, the neonate’s father found him unconscious on his bed in a dorsal position. Despite several attempts at resuscitation, by the father first and then by the emergency staff, the neonate was declared dead. An autopsy was requested and performed within 24 hours of death. The gross macroscopic investigation revealed that both lungs were mildly edematous, the brain mildly swelled, and adrenal glands were normal, with no signs of hemorrhage found.
The coroner together with the forensic microbiologist performed sampling of cardiac and peripherical blood, lungs, heart, kidney, liver, intestinal matter, brain and cerebrospinal fluid (turbid) for microbiological examinations. All samples were analyzed for bacterial detection, and intestinal matter, blood and cerebrospinal fluid were also collected to detect viral agents. Nasal and pharyngeal swabs were also taken. All specimens were handled following Riedel’s recommendations, using dedicated instruments and iodine scrubs to sterilize whole body surfaces [19]. Tissue samples, homogenized in sterile PBS, and swabs underwent culture analysis using both nonselective and selective media (Columbia blood agar, Chocolate agar, MacConkey agar, Sabouraud agar, Mannitol agar), that were incubated under aerobic and anaerobic atmosphere at 37° up to seven days. The blood and cerebrospinal fluid were inoculated into a Bactec blood culture system (Beckton Dickinson) using pediatric bottle and incubated for seven days. Except for the intestinal sample, after 48 hours of incubation all specimens were positive for Gram-positive, catalase-negative, cocci grown as pure culture onto non-selective and selective blood-based agars (Figure 1). They were identified as Streptococcus agalactiae by Vitek2 GP card (bio–Mérieux, France) and subsequently confirmed by matrix-assisted laser desorption ionization-time of flight mass spec–trometry (MALDI-TOF MS) using Bruker Biotyper software 2.0 (Bruker Daltonics, Germany). No viral agents were found.
Antimicrobial susceptibility testing was performed by Vitek 2.0 (bioMérieux) revealing the strain was susceptible to all antibiotics tested but tetracycline (Table 1).
Antibiotic
MIC (µg/ml)
S/I/R (EUCAST)
S/I/R (CLSI)
Penicillin G
= 0.12
S
S
Clindamycin
= 0.25
S
S
Levofloxacin
1
S
S
Linezolid
2
S
S
Moxifloxacin
= 0.25
S
NA
Teicoplanin
= 0.5
S
NA
Tetracycline
³61
R
R
Tigecyclin
= 0.12
S
NA
Trimethoprim/Sulfamethoxazole
=10
S
NA
Vancomycin
= 0.5
S
S
Table 1: Histologic assessment of post-mortem lung tissue. Lung sections were stained by hematoxylin-eosin staining. Representative sections illustrating lung inflammation for each group are shown. Magnification: ×4 (A) and ×10 (B).
Histologic examination of lungs revealed the presence of bacterial clusters in vessels and inside alveolar spaces (Gram staining), and a marked neutrophilic inflammation involving about 90% of the alveolar compartment (hematoxylin-eosin staining) (Figure 1).
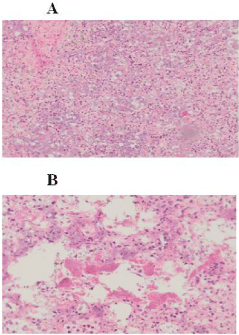
Figure 1: Histologic assessment of post-mortem lung tissue. Lung sections
were stained by hematoxylin-eosin staining. Representative sections
illustrating lung inflammation for each group are shown.
Magnification: ×4 (A) and ×10 (B).
Discussion
Group B Streptococcus (GBS) is an important pathogen causing neonatal meningitis and pneumonia [3]. Most cases of EOD present with bacteremia without a focus, where respiratory, neurological and cardiovascular signs are the main initial signs [2]. More severe cases of EOD may be characterized by shock and severe respiratory distress failure at birth, although mild, non-specific symptoms may occur [20].
The main risk factors (RFs) recognized to increase the likelihood of EOD in a neonate born to a mother colonized by GBS include GBS bacteriuria during pregnancy (the only one in 50-60% of EOD cases), a previous invasive GBS infection, amniotic membrane’s premature rupture more than 24 hours before vaginal delivery, intrapartum maternal temperature over 38°C, and preterm labor or membrane rupture before 37 weeks’ gestation [21].
The present case report describes a GBS infection in a term neonate without any congenital pulmonary or circulatory malformations. A recent study from Italy reported that neonates exposed to IAP were significantly more likely to present signs of illness at birth, as opposed to neonates unexposed to IAP [22], and this could explain the absence of signs or symptoms found in this neonate with no administration of IAP of the mother.
GBS, a commensal of human intestine and recto-vaginal tract, is classified into nine serotypes, all able to cause infants’ disease although specific serotypes, mostly III, Ia, V, Ib and II, and clones (CC17) are more frequently detected [13]. Unfortunately, in the present case the GBS strain was not available for further characterization to determine the specific serotype and clone.
The prevalence of the colonization in pregnant women ranges from 10 to 30% [1,2], according to age, ethnicity, body site sampled and microbiological tests. In particular, heavy bacterial colonization of the mother has been reported to represent the highest risk of perinatal transmission [23]. Culture-based screening methods using vaginal and anorectal swabs of all pregnant women at 35-37 weeks of gestation is recommended to identify those who should receive IAP [21,24].
Here, a sudden death of an infant with a post-mortem diagnosis of EOD caused by GBS has been reported with a negative vaginorectal screening of the mother. The mother did not receive antibiotics during the gestation period and the negative culture for GBS perinatal screen did not induce the administration of any antibiotic during labor. To date, culture is considered the gold standard, although questions have arisen concerning their positive and negative predictive value in late pregnancy [9,25-27]. False-negative or -positive results could have important implications in cases of EOD in neonates born to culturenegative mothers and can also lead to unnecessary antibiotics in culture-positive women whose status changes just before delivery [28]. In our case, the negative result of GBS screening by culture methods could be solved by using more specific and sensitive methods. Rapid, reliable and accurate (both sensitivity and specificity> 90%), Nucleic Acid Amplification Tests (NAATs) have been recently introduced to detect GBS carriage status, especially in the delivery room [26,29]. Although more expensive than standard antenatal cultures, NAATs could avoid unnecessary treatment of women with positive screening that become negative at delivery screening, thus resulting in being also cost-saving methods [30,31].
The infection of GBS are relatively rare in Italy [22], with an incidence (0.18 per 1000 live birth) lower than USA (0.40 per 1000 live birth), but slightly higher than to the Japan (0.10 cases for 1000 live birth).
Sudden unexpected infant’s death is one of the most important matters in forensic medicine and making a differential diagnosis between internal/infection and external/violent death is of paramount importance. In our case, the post-mortem microbiological diagnosis has been an essential tool for understanding the nature of unexpected death to determine the etiology of disease with the isolation of a GBS strain that remained hidden. Although until today the post-mortem microbiology remains a controversial topic, in our case it proved to be indispensable for reaching the correct cause of the infant’s death due by a streptococcal infection.
Acknowledgement
We gratefully thank Sherry Lynn Jones (EdD, MS, RN; Faculty, International Critical Incident Stress Foundation; MD, USA) for providing language editing.
References
- Almeida A, Villain A, Joubrel C, Touak G, Sauvage E, Rosinski-Chupin I, et al. Whole-genome comparison uncovers genomic mutations between group B streptococci sampled from infected newborns and their mothers. J Bacteriol. 2015; 197: 3354-3366.
- Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008; 299: 2056-2065.
- Zangwill KM, Schuchat A, Wenger JD. Group B streptococcal disease in the United States, 1990: report from a multistate active surveillance system. MMWR CDC SurveillSumm. 1992; 41: 25-32.
- Kalin A, Acosta C, Kurinczuk JJ, Brocklehurst P, Knight M. Severe sepsis in women with group B streptococcus in pregnancy: an exploratory UK national case-control study. BMJ Open. 2015; 5: e007976.
- Berardi A, Cattelani C, Creti R, Berner R, Pietrangiolillo Z, Margarit I, et al. Group B streptococcal infections in the newborn infant and the potential value of maternal vaccination.Expert Rev Anti Infect Ther. 2015; 13: 1387-1399.
- El Beitune P, Duarte G, Leite Maffei CM. Colonization by Streptococcus agalactiae during pregnancy: maternal and perinatal prognosis. Braz J Infect Dis. 2005; 9: 276-282.
- Chung MY, Ko DJ, Chen CC, Huang CB, Chung CH, Chen FS, et al. Neonatal group B streptococcal infection: a 7-year experience. Chang Gung Med J. 2004; 27: 501-508.
- Baker CJ, Edwards MS. Group B Streptococcus infections. Remington JS, Klein OJ, editors. In: Infectious disease of the fetus and newborn infant. 4th ed. Philadelphia, PA: WB Saunders. 1995.
- Barbadoro P, Marigliano A, Savini S, D’Errico MM, Prospero E. Group B streptococcal sepsis: an old or ongoing threat? Am J Infect Control. 2011; 39: e45-48.
- MacFarquhar JK, Jones TF, Woron AM, Kainer MA, Whitney CG, Beall B, et al. Outbreak of late-onset group B Streptococcus in a neonatal intensive care unit. Am J Infect Control. 2010; 38: 283-288.
- Easmon CS, Hastings MJ, Clare AJ, Bloxham B, Marwood R, Rivers RP, et al. Nosocomial transmission of group B streptococci. Br Med J. (Clin Res Ed) 1981; 283: 459-461.
- Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AK, Cousens S, et al. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet. 2012; 379: 547-556.
- Imperi M, Gherardi G, Berardi A, Baldassarri L, Pataracchia M, Dicuonzo G, et al. Invasive neonatal GBS infections from an area-based surveillance study in Italy. ClinMicrobiol Infect. 2011; 17: 1834-1839.
- Schrag SJ, Zywicki S, Farley M, Reingold AL, Harrison LH, Lefkowitz LB, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. New Engl J Med. 2000; 342: 15-20.
- Schrag SJ, Zell ER, Lynfield R, Roome A, Arnold KE, Craig AS, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med. 2002; 347: 233-239.
- Center for Disease Control and Prevention. Perinatal group B streptococcal disease after universal screening recommendations - United States 2003- 2005. 2007; 56: 701-705.
- Kwatra G, Cunnington MC, Merrall E, Adrian PV, Ip M, Klugman KP, et al. Prevalence of maternal colonisation with group B streptococcus: a systematic review and meta-analysis. Lancet Infect Dis. 2016; 16: 1076-1084.
- Fernández-Rodríguez A, Cohen MC, Lucena J, Van de Voorde W, Angelini A, Ziyade N, et al. How to optimise the yield of forensic and clinical post-mortem microbiology with an adequate sampling: a proposal for standardisation. Eur J Clin Microbiol Infect Dis. 2015; 34: 1045-1057.
- Riedel S. The value of postmortem microbiology cultures. J Clin Microbiol. 2014; 2: 1028-1033
- Edwards MS, Nizet V. Group B streptococcal infections. Remington JS, Klein JO, editors. In: Infectious diseases of the fetus and newborn infant. 8th edition. Elsevier Saunders; Philadelphia: 2016. P411-456.
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. 2010. MMWR Recomm Rep. 2010; 59: 1-36.
- Berardi A, Lugli L, Rossi C, China M, Chiossi C, Gambini L, et al. Intrapartum antibiotic prophylaxis failure and group-B streptococcus early-onset disease. J Matern Fetal Neonatal Med. 2011; 24: 1221-1224.
- Regan JA, Klebanoff MA, Nugent RP, Eschenbach DA, Blackwelder WC, Lou Y, et al. Colonization with group B streptococci in pregnancy and adverse outcome. VIP Study Group. Am J Obstet Gynecol. 1996; 174: 1354-1360.
- Tibussek D, Sinclair A, Yau I, Teatero S, Fittipaldi N, Richardson SE, et al. Late onset group B streptococcal meningitis has cerebrovascular complications, J Pediatr. 2015; 166: 1187-1192.
- Berardi A, Lugli L, Rossi C, Guidotti I, Lanari M, Creti R, et al. Impact of perinatal practices for early-onset group-B streptococcal disease prevention. Pediatr Infect Dis J. 2013; 32: 265-271.
- El Helali N, Nguyen JC, Ly A, Giovangrandi Y, Trinquart L. Diagnostic accuracy of a rapid real-time polymerase chain reaction assay for universal intrapartum group B streptococcus screening. Clin Infect Dis. 2009; 49: 417- 423.
- Pulver LS, Hopfenbeck MM, Young PC, Stoddard GJ, Korgenski K, Daly J, et al. Continued early onset group B streptococcal infections inthe era of intrapartum prophylaxis. J Perinatol. 2009; 29: 20-25.
- Berardi A, Rossi C, Guidotti I, Vellani G, Lugli L, Bacchi Reggiani ML, et al. Factors associated with intrapartum transmission of group B streptococcus. Pediatr Infect Dis J. 2014; 33: 1211-1215.
- McKenna JP, Cox C, Fairley DJ, Burke R, Shields MD, Watt A, et al. Loopmediated isothermal amplification assay for rapid detection of Streptococcus agalactiae (group B streptococcus) in vaginal swabs - a proof of concept study. J Med Microbiol. 2017; 66: 294-300.
- Bergeron MG, Danbing K. New DNA-based PCR approaches for rapid realtime detection and prevention of group B streptococcal infections in newborns and pregnant women. Reprod Med Rev. 2004; 11: 25-41.
- El Helali N, Giovangrandi Y, Guyot K, Chevet K, Gutmann L, Durand-Zaleski I. Cost and effectiveness of intrapartum group B streptococcus polymerase chain reaction screening for term deliveries. Obstet Gynecol. 2012; 119: 822- 829.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 26th ed. CLSI supplements M100S. Wayne, Pennsylvania. USA. 2016.
