Abstract
Lateral Flow Assays (LFAs) are devices that have acquired a great popularity worldwide because of their ergonomic design; do not require refrigeration for storage. As well as, are easy to use, this facilitates its manipulation by non-specialized personnel; provide results in a short period, which is very advantageous in modern times. In addition, these tests can be acquired by people of low economic resources, due to their relatively low costs; they provide very reliable results and have great sensitivity and specificity; among many other positive properties. The LFA format consists of several components: 1) sample pad is the placement area of the sample; 2) conjugate pad contains the component of interaction with the positive sample associated with the reporter labels; 3) nitrocellulose membrane chemically treated to produce a test line and/or control line, which are the indicators of a positive or negative sample. The reporter labels are varied: colloidal gold, enzymes, colored latex beads, quantum dots, carbon nanoparticles and their use depends on the requirements of each test in particular. This review focuses on the general aspects of these rapid tests of great utility in multiple areas of life such as: clinical diagnosis, veterinary, agriculture, biowarfare, food analysis, environmental health and safety, industrial testing, as well as in new areas that have arisen more recently, such as molecular diagnostics and theranostics
Introduction
Lateral flow assays (LFA), also called Rapid Test Devices (RTD), are tools based on chromatography whose principle is very simple: the test sample is placed in the sample pad to determine the presence or absence of the particular analyte, then the displacement lateral occurs through capillary action to reach the conjugate pad where the analyte binds to the reporter label, these molecules continue to migrate until they come the Control Lines (LC) and the Test Line (TL), then the liquid continues travel until the absorbent pad. The LC is an internal control of these tests and must always provide a signal (which allows corroborating the correct operation) and TL indicates if the sample is positive (presence of signal) or negative (absence of signal); in the case of a positive sample this process promotes an interaction chemical that generates a signal indicative of reaction, such as the appearance of color [1].
Some outstanding advantages and disadvantages of LFA are shown in Table 1.
Advantages
Disadvantages
The design is very simple and easy to produce industrially
Sometimes has been detected lack of reproducibility
The interpretation of results is simple. Signal on/off
Assays requiring high analytical sensitivity
Allow the analysis of the samples in a single step, generally the tests are fast (»5 min)
Visual interpretation generates inconveniences when a very slight signal is produced
Economic
Occasionally the use of a traditional reporter does not produce a signal
Stable. Shelf-lives of 12-24 months often without refrigeration
Ease of transport
Do not require specialized personnel or equipment, for this reason can be employed in the field
Enables the evaluation of multiple analytes in a simple device
Tools very versatile and flexible
Table 1: Advantages and disadvantages of LFA.
In the year 1950 was developed the first dipstick to detect glucose in urine and determine the presence of diabetes in the evaluated individuals and 10 years after the market launch was made for commercialization. The results obtained after performing the urine evaluation are analyzed by means of a colourimetric chart that allows the determination of the glucose concentration [2]. After the appearance of this great technological development, the dipsticks have been adapted to the detection of a huge quantity of analytes and due to its innumerable advantages, billions of tests are produced during each year. Another of the applications that have driven the design of lateral flow tests is the detection of pregnancy in humans, of great importance at the world medical level, supported with the research carried out by Vaitukaitis et al [3] which are the great pioneers in the studies on the determination of human Chorionic Gonadotropin (hCG). Lateral-flow assays have been used as diagnostic tools for monitoring toxins [4], hormones [5], drugs of abuse [6] and pathogens [7-9] among others, which favors a detection of analytes qualitative. This type of tests are ideal for use in critical and unprotected areas, where there are economic limitations such as rural health centers, as well as hospital centers located in poor neighborhoods that have not specialized staff or equipment [10].
Structure of LFA
Pad for sample application
It is the support where the sample is placed to perform the evaluation. This type of test allows the evaluation of a variety of samples, such as: blood from a mother after childbirth, sputum from a possible tuberculosis patient, or a sample of ground beef from a bulk container. The manufacturing materials more employed are cellulose acetate and/or glass fiber because cellulose acetate membrane shows low affinity towards proteins and glass fiber membrane shows no affinity towards proteins [11]. The role of the sample pad is to act as a sample absorber, so that it is compatible with the assay, distribute it homogeneously, release it to migrate laterally with high efficiency and control the flow of the sample to the conjugate pad. This component must contain buffer salts, proteins, surfactants and other liquids to control the flow rate of the sample and to make it suitable for the interaction with the detection system [12]. Moreover, the pores of the sample pad can act as a filter that removes contaminants (e.g. red blood cells) and allow obtaining a cleaner sample, which increases the quality of the assay.
Pad of conjugate
The main role of this component is to keep the dry conjugate stable over time and allow it to be released quickly, efficiently, uniformly and reproducibly during the evaluation [13], in order to obtain excellent signals in control line and test line. The materials used to manufacture this component include cellulose, glass fiber, polyesters, polypropylene or polyethylene, which show low nonspecific binding. During the preparation of the conjugate are added carbohydrates that act as stabilizing agents that allow to preserve their biological structure [14], also at the time of performing the assay, they work as diluent compounds that transport the conjugate through the LFA to favor a reproducible path when compare the test strips. The placement of the conjugates on the pad should be done very carefully, often using two methods: a) immersion of the pad in the conjugate solution together with a solution containing proteins, surfactants and polymers to then proceed to drying, b) placement using dispensers such as BioDot AirJet Quanti 3000 that apply the aerosol compound [15].
The labels most commonly used include colloidal gold, latex particles, visual or fluorescent dye. Among these tags colloidal gold it is one of the most outstanding due to its easy preparation, it is economical, has an intense color that presents a very convenient and forceful visualization, it is very stable, the signal remains for a long time after that the membrane it is colored [16]. It is essential that the conjugates are stable throughout the validity period of the test to avoid erroneous results, therefore, compounds should be added that favor the maintenance of their structure, which promotes the reproducibility and efficient release of the conjugate pad.
Nitrocellulose membrane
This component is the solid support of LFA, constitutes a critical and crucial point in the sensitivity of the dipstick. In the market there are nitrocellulose membranes with different pore sizes, which are used according to the needs of each test in particular; in case of requiring great sensitivity and a slow flow rate, membranes with small pore size are used, however, for fast tests and tests that require less sensitivity, membranes with large pore size are used. The test and control line are dispensed on this material, to subsequently carry out the blockade, which must be very efficient to avoid non-specific interactions. This material offers several advantages: it is relatively inexpensive, has high affinity for some biomolecules (e.g. proteins) and is easy to handle [17].
Absorbent pad
It is located at the end of the device and is usually designed with cellulose fiber. The function of this component is to maintain a uniform capillary flow through the membrane in the correct direction, at an adequate flow rate and to collect the excess liquid used in the LFA [18].
Backing laminate
The LFA components are assembled in a case made of materials such as polyester, Polyvinyl Chloride (PVC), or mixtures of both components. This device contains an adhesive that must be soft to generate enough adhesion and very strong to avoid its transfer to the test components.
A type of LFA widely used in the world is Lateral Flow Immunoassay (LFIA). In this variant the white antibodies present in the serum of the positive patient migrate and interact with the reporter (eg colloidal gold conjugated protein A), then they bind to the antigen that is adsorbed in the nitrocellulose line test and provide a purple line indicative of positive reaction.
The standard composition of an LFIA and the development of the assay can be observed in Figure 1.
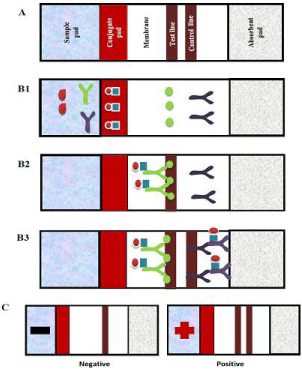
Figure 1: A) Components of LFA. Development of an immunoassay; B)
Placement of the drop of blood or serum that migrates laterally; B1) The
antibodies are bound by the Fc region to protein A (■)/colloidal gold (●). B2) In
case of a positive sample in the test line it is produce the interaction antigenantibody,
which causes precipitation of colloidal gold. B3) Nonspecific
antibodies (Y)) Continue to migrate and interact with anti-IgG antibodies (Y)
and are deposited in the control line; C) A negative sample produces a band
that corresponds to the control line, a positive sample originates two bands in
the test line and the control line.
Applications of LFA
The process of development of LFA obtained its first fruits in the late 1980s when began to be marketed, whose application has expanded to the step of giants including the following fields: clinical diagnostics, veterinary, agriculture, biowarfare, food, environmental health and safety, industrial testing, as well as newer areas such as molecular diagnostics and theranostics. Here are some examples of rapid tests used throughout the world that have allowed us to solve multiple problems in different areas of our daily lives. Escandón et al [19] evaluated the quality of the LFA CrAg against 421 samples of sera with a positive diagnosis of HIV from the Epidemiology and Infectious Diseases Group of the Erasmo Meoz University Hospital (HUEM), Cúcuta (Colombia); In addition, they made a comparison of the results obtained with CrAg Latex. Cryptococcosis is a common opportunistic infection in people with AIDS, especially those who have limited access to Highly Active Antiretroviral Therapy (HAART) [20]. In a large study conducted in Colombia, only 37% of people with AIDS and cryptococcosis were alive 6 months after diagnosis and, of these, a significant number of neurological symptoms, due to these reasons is of great medical importance [21]. It was demonstrated that CrAg LFA shows greater sensitivity than CrAg Latex; This has also been observed by the manufacturers who have determined that the sensitivity of CrAg LFA is 1 ng CrAg/ml, while the CrAg Latex Meridian CALAS has a sensitivity of 19 ng CrAg/ml. This experience shows that rapid tests are essential in the detection of infectious diseases, such as the CrAg LFA, which allows the detection of cryptococcosis during its initial stages in HIV positive patients and provides them with a better quality of life. Sastre et al [22] have developed a lateral duplex flow assay for simultaneous detection of antibodies against African and Classical swine fever viruses, an infection that affects domestic and wild pigs of all breeds and ages, causing a wide range of syndromes from mild disease to lethal hemorrhagic fever [23]. This test recognizes anti-ASF (African swine fever) and anti-CSF (Classical swine fever) in the sera evaluated, based on the use of colored carboxyl-modified latex microspheres that are covalently linked to specific antigens of these viruses: VP72 protein of ASFV and E2 protein of CSFV. VP72 was linked to red latex particles and E2 was bound to blue latex particles and green latex particles were bound to biotin which are placed on the conjugate pad. The test line 1 contains VP72, the test line 2 contains E2 and the control line contains antibiotin IgG monoclonal Antibody (mAb). When a negative sample is placed, occurs a lateral displacement that produces the interaction biotin-green latex/mAb that causes a green color in the control line; in a positive sample for ASFV the anti-VP72 antibodies bind to VP72-latex red, they migrate and VP72 binding occurs in the test line that produces a red color; in a positive sample for CSFV the anti-E2 antibodies bind to E2-blue latex, move laterally until they reach the E2-containing line where a blue color is produced. The results are interpreted as follows: green signal means negative, red signal means positive for ASFV, blue signal indicates positive for CSFV, red signal and blue means positive for ASFV and CSFV. The diagnosis is effective, fast, very easy to use in the field and can be done from the first two weeks of infection, which is essential to reduce the spread of the virus to healthy animals. Rong-Hwa et al [24] performed the detection of Staphylococcal Enterotoxin B (SEB), a toxin that triggers food poisoning that causes diarrhea, vomiting and/or nausea [25] and has been considered as an attractive choice for a biological weapon spray to contaminate water or food sources due to certain particular properties such as its great stability, easy propagation, great toxic effects and high morbidity [26]. For these reasons, it was decided to develop an immunochromatographic assay that allows detecting SEB toxin in biological fluids. Samples containing the toxin migrate in the membrane and bind in the conjugate pad to SEB IgG-coated colloidal gold nanoparticles, which are then captured by the anti-SEB antibody (test line) and then bind to the animal’s specific IgG evaluated (test control), which produces two lines of intense red color. In the case of a negative sample, only a single line of color corresponding to the line test is detected. This test allows to determine the presence of SEB toxin in a very specific, sensitive, cost-effective manner and provides results in a short time (≈5 min). The implementation of LFA in food safety assessments has increased, to the point that trials have been marketed for the evaluation of contaminants. Smiths et al [27] developed a test immunochromatographic Brucella-Specific Immunoglobulin M and G Lateral Flow Assays for rapid serodiagnosis of human brucellosis. The detection strip contains Brucella Lipopolysaccharide (LPS) as a Brucella-specific capture probe as well as a reagent control applied in distinct lines. The reagent pad contains dried and stabilized detection reagent consisting of a colloidal gold immune conjugate. IgM was detected using colloidal gold-conjugated anti-human IgM and IgG by using colloidal gold-conjugated anti-human IgG was used as detection reagent in the IgG flow assay. This trial shows a sensitivity of Brucella IgM and IgG calculated for the combined assay results is 96%, and specificity of 99%, can be used to detect acute, persistent, relapsing disease, as well as to monitor chemotherapeutic treatment.
Other types of tests widely used include amperometric blood glucose tests [28], color-indicator urine dipsticks [29], and detection of Human Immunodeficiency Virus (HIV) [30].
Conclusion
LFA has been used in numerous investigations involving the detection of clinical and non-clinical analytes. This platform is widely accepted and demanded worldwide because they are simple procedures, use very small sample volumes, generate results very quickly, present low cost, do not require specialized personnel or equipment, the results are easy to interpret, they have a long shelf life and they do not require refrigeration for their storage, it is versatile since it can use variety of reporter label depending on the needs of each dipstick; security because its sensitivity and specificity are excellent; it is practical because it allows the evaluation of several analytes in a single test. Due to all these characteristics are very well adapted for use in any individual regardless of their geographic zone of origin: from developing countries, small ambulatory care settings, to remote regions and battlefields. The dipstick technology has a very simple functioning: the test sample is placed on the sample pad, migrates by capillary diffusion until reaching the conjugate pad, the developed complexes are released and reach the detection zone where the lines are located control and test line, then The solution follows its displacement until the absorbent pad where the liquid is absorbed.
The ultimate goal is to achieve the optimization of the dipstick. Obtaining high quality products allows us to improve the diagnosis of innumerable diseases. Which leads to a rapid implementation of chemotherapeutic treatments. As well as, great achievements can be generated in the rapid and efficient detection of analytes, such as toxins or drugs.
References
- Koczula K, Gallotta A. Lateral flow assays. Essays Biochem. 2016; 60: 111- 120.
- Yetisen A, Akram M, Lowe C. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip. 2013; 13: 2210-2251.
- Vaitukaitis J, Braunstein G, Ross G. A radioimmunoassay which specifically measures human chorionic gonadotropin in the presence of human luteinizing hormone. J Obstet Gynecol. 1972; 15: 751-758.
- Shyu R, Shyu H, Liu H, Tang S. Colloidal gold-based immunochromatographic assay for detection of ricin. Toxicon. 2002; 40: 255-258.
- You D, Park T, Yoon J. Cell-phone-based measurement of TSH using Mie scatter optimized lateral flow assays. Biosens Bioelectron. 2013; 40: 180- 185.
- Carrio A, Sampedro C, Sanchez-Lopez J, Pimienta, M, Campoy P. Automated low-cost smartphone-based lateral flow saliva test reader for drugs-of-abuse detection. Sensors (Basel). 2015; 15: 29569-29593.
- Al-Yousif Y, Anderson J, Chard-Bergstrom C, Kapil S. Development, evaluation, and application of lateral-flow immunoassay (immunochromatography) for detection of rotavirus in bovine fecal samples. Clin Diagn Lab Immunol. 2002; 9: 723-725.
- Barfield C, Barney R, Crudder C, Wilmoth J, Stevens D, Mora-Garcia S, et al. A highly sensitive rapid diagnostic test for Chagas Disease that utilizes a recombinant Trypanosoma cruzi antigen. IEEE Trans Biomed Eng. 2011; 58: 814-817.
- Gwyn S, Mitchell A, Dean D, Mkocha H, Handali S, Martin D. Lateral flowbased antibody testing for Chlamydia trachomatis. J Immunol Methods. 2016; 435: 27-31.
- Pai N, Pai M. Point-of-care diagnostics for HIV and tuberculosis: landscape, pipeline, and unmet needs. Discov Med. 2012; 13: 35-45.
- Bangs Laboratories. Lateral Flow Tests, Inc. TechNote. 2013; 303:1-6.
- Millipore. Rapid Lateral Flow Test Strips Considerations for Product Development. 2008.
- Millipore Corporation. Rapid Lateral Flow Test Strips: Considerations for Product Development. Lit. No. TB500EN00. Bedford, MA. 2002.
- Anon. Rapid Lateral Flow Test Strips: Considerations for Product Development, Merck Millipore, Billerica. 2008.
- Leung W, Chan P, Bosgoed F, Lehmann K, Renneberg I, Lehmann K, et al. One-step quantitative cortisol dipstick with proportional reading. J Immunol Methods. 2003; 281: 109-118.
- Cvak B, Pum D, Molinelli A, Krska R. Synthesis and characterization of colloidal gold particles as labels for antibodies as used in lateral flow devices. Analyst. 2012; 137: 1882-1887.
- Sajid M, Kawde AN, Daud M. Designs, formats and applications of lateral flow assay: a literatura review. J Saudi Chem Soc Journal. 2015; 19: 689-705.
- O’Farrell B. Evolution in lateral flow-based immunoassay systems, in Lateral Flow Immunoassay. Humana Press. 2009; 1-33.
- Escandón P, Lizarazo J, Agudelo C, Chiller T, Castañeda E. Evaluation of a rapid lateral flow immunoassay for the detection of cryptococcal antigen for the early diagnosis of cryptococcosis in HIV patients in Colombia. Medical Mycology. 2013; 51: 765-768.
- Park B, Wannemuehler K, Marston B, Govender N, Pappas P, Chiller T. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009; 23: 525-530.
- Lizarazo J, Linares M, de Bedout C, Restrepo A, Agudelo C, Castañeda E. Grupo Colombiano para el Estudio de la Criptococosis. Clinical and epidemiological study of cryptococcosis in Colombia: results of nine years of the national survey, 1997-2005. Biomedica. 2007; 27: 94-109.
- Sastre P, Pérez T, Costa S, Yang X, Räber A, Blome S, et al. Development of a duplex lateral flow assay for simultaneous detection of antibodies against African and Classical swine fever viruses. J Vet Diagn Invest. 2016; 28: 543- 549.
- Blome S, Gabriel C, Beer M. Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res. 2013; 173: 122-130.
- Rong-Hwa S, Shiao-Shek T, Der-Jiang C, Yao-Wen H. Gold nanoparticlesbased lateral flow assay for detection of staphylococcal enterotoxin B. Food Chem. 2010; 118: 462-466.
- Evenson M, Hinds W, Berstein R, Bergdoll S. Estimation of human dose of staphylococcal enterotoxin A from a large outbreak of staphylococcal food poisoning involving chocolate milk. Int J Food Microbiol. 1988; 7: 311-316.
- Poli M, Rivera V, Neal D. Sensitive and specific colorimetric ELISAs for Staphylococcus aureus enterotoxins A and B in urine and buffer. Toxicon. 2002; 40: 1723-1726.
- Smiths H, Abdoel T, Solera J, Clavijo E, Diaz R. Immunochromatographic Brucella-Specific Immunoglobulin M and G Lateral Flow Assays for Rapid Serodiagnosis of Human Brucellosis. Clin Diagn Lab Immunol. 2003; 10: 1141-1146.
- Newman J, Turner A. Home blood glucose biosensors: a commercial perspective. Biosens Bioelectron. 2005; 20: 2435-2453.
- Luntz G. A simple quick test for glucose in urine. BMJ. 1957; 1: 499-500.
- Greenwald J, Burstein G, Pincus J, Branson B. A rapid review of rapid HIV antibody tests. 2006; 125-131.
