Research Article
Austin Clin Neurol 2014;1(1): 1003.
Neuropsychological Outcomes in Multiple Sclerosis Patients Treated with Deep Brain Stimulation for Tremor
Gareth Davies1, Ki Chang1, Elana Farace1, Jonas Sheehan1 and James McInerney1*
Department of Neurosurgery, Pennsylvania State University, USA
*Corresponding author: James McInerney, Department of Neurosurgery, Pennsylvania State University, College of Medicine and Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033, USA
Received: January 10, 2014; Accepted: January 17, 2014; Published: January 28, 2014
Abstract
Background: Multiple sclerosis (MS) can often produce a severe cerebellar outflow tremor that is typically poorly responsive to medical management. Deep brain stimulation (DBS) targeted to the ventral intermediate nucleus (VIN) of the thalamus has been shown to suppress the tremor, but long–term outcomes for tremor control in the MS population remain unclear. Furthermore, neuropsychological outcomes for this population have not previously been well described, particularly following DBS.
Objectives: The purpose of this study was to assess the long–term outcome of tremor control in our MS patient population following DBS as well as to describe any potential changes in standard neuropsychological measures.
Methods: A series of eight patients underwent unilateral or bilateral VINthalamic DBS implantation for control of MS related tremor and were followed for a mean of 30.5 months. Postoperative tremor control and pre and postoperative neuropsychological data were retrospectively reviewed.
Results: All patients subjectively reported improvement in tremor with stimulation at the most recent available follow–up. Review of neuropsychological outcomes revealed no significant deterioration postoperatively. Conclusions: DBS may improve tremor and decrease disability in the MS population, and does not appear to cause deterioration in neuropsychological outcomes. DBS may be an important adjunct to therapy for MS related tremor.
Keywords: Multiple sclerosis; Tremor; Deep brain stimulation; Neuropsychological outcomes
Introduction
Multiple sclerosis (MS) is the most common disease caused by an inflammatory demyelinating process in the central nervous system. High–frequency areas of the world, including the northern United States, have a disease prevalence of 85 per 100,000 people [1]. Because MS has only a modest negative effect upon longevity but potential for considerable disability over many years, the socioeconomic consequences of this disease are dramatic [2]. Of the wide array of symptoms seen in this disease, tremor is one of the most disabling and difficult to treat. Tremor affects 25% to 58% of patients with MS [3], and is characterized as disabling in 3–15% of patients [4]. The typical tremor is a cerebellar outflow tremor [4], characterized as an intention tremor, and this most commonly affects the upper extremities [3]. Tremor is particularly pronounced during the most demanding aspects of precise movements, and typically occurs at 2–3 Hz. Mild appendicular MS tremors may be treated with weighted wrist bracelets or specialized utensils, but tremor typically progresses as the disease progresses. Attempts at pharmacological management of the tremor typically involve isoniazid, pyridoxine, primidone, carbamazepine, gabapentin, clonazepam, or propranolol [2]. These treatments generally have marginal success at tremor control, and side effects are often intolerable [2]. Therefore, attention has turned to surgical management of disabling MS tremor. Early surgical therapy has consisted of ablative thalamotomy [4], but this therapy cannot be modulated over time and its use has therefore become limited [5], with attention turning to deep brain stimulation for primary treatment of MS tremor.
Patients with multiple sclerosis are not only affected by disabling tremor, however, but may also suffer from a myriad of neuropsychiatric conditions. Most prominent are depression, fatigue, cognitive problems, sleep disorders and disorders of sexual function [6]. Epidemiologic studies have shown that 50% of MS patients will develop major depression within their life time, compared to 8% of the general population. Furthermore, 15% will experience an episode of major depression in any given year, which is approximately three–times higher than the general population. Other studies have demonstrated that atypical features of depression such as irritability and anger may predominate over classic symptoms of diminished mood or anhedonia, rendering care giving more difficult [6]. Cognitive difficulties affect at least 50% of patients suffering from MS and often mirror the symptoms of subcortical dementia. Most notable deficits involve tasks emphasizing retrieval from recent memory stores, information processing speed, and working memory, with relative sparing of language and intellectual function [7]. Brassington and Marsh demonstrated that approximately 30% of MS patients have substantial memory dysfunction and another 30% have moderate memory problems. Patients often complain of difficulty remembering conversations, appointments and details of work tasks as well as decreased ability to immediately recall verbal or visual information [8]. Furthermore, MS affects attention, concentration, and speed of information processing, which diminishes effectiveness in tasks involving working memory, attention switching, or rapid visual scanning demands. Compared to healthy controls, MS patients have a decrease in visual processing speed demonstrated by the symbol digit modalities test (SDMT), one of the most sensitive tasks to detect cognitive impairment in MS. Also, approximately 15–25% of individuals with MS show substantial difficulties in executive function, involving cognitive flexibility, concept–formation, verbal abstraction, problem–solving, inhibitory control, planning, and verbal fluency [8].
With the notable detrimental effect that both tremor and neuropsychiatric complications have upon the quality of life for patients with multiple sclerosis, attention may be turned to surgical management of tremor with a focus upon ensuring that surgery does not exacerbate neuropsychiatric deterioration.
Thalamotomy was once the first line option when surgical therapy was considered to treat MS tremor. Recent data suggests that efficacy in tremor control is equivocal between DBS and thalamotomy, with DBS having the advantage of reversibility and adjustability [9]. Although adverse effects can occur with both procedures, neuropsychiatric complications have not been fully elucidated with either. A six month comparison of the neuropsychological effects of thalamotomy and thalamic stimulation in patients with severe drug–resistant tremor due to Parkinson’s disease (PD), essential tremor (ET), or MS found that both treatment options were associated with a minimal overall risk of cognitive deterioration, although verbal fluency decreased after both left–sided thalamotomy and thalamic stimulation. Interestingly, DBS was associated with a greater improvement in the state of anxiety and mood. It should also be noted that thalamotomy had a substantially higher morbidity than thalamic stimulation, mainly involving dysarthria and motor function [10] as well as permanent hemiparesis and seizures [11].
Deep brain stimulation (DBS) of the ventral intermediate nucleus (VIN) of the thalamus is a well–established, generally effective treatment option for patients with medication–resistant tremor associated with PD or ET [3]. VIN–thalamic DBS has also been used for control of MS related tremor, and a lower complication rate than that seen with thalamotomy has been suggested [11]. However, longterm outcome reports have been limited in number [4], have indicated varying degrees of success with DBS [3], or have included patients who underwent thalamotomy as well as including DBS patients in their analysis [11, 4]. Furthermore, neuropsychological outcomes for this population have not previously been well described.
In the current report, a series of eight patients who underwent unilateral or bilateral VIN–thalamic DBS implantation for control of MS related tremor were reviewed from a prospectively collected clinical database. Long–term outcomes for tremor control as well as for neuropsychological measures were analyzed.
Methods
A series of eight patients treated for tremor and also diagnosed with multiple sclerosis were identified from the quality improvement database maintained for all deep brain stimulation patients at our institution. The data reviewed originated from standard clinical practice carried out for all deep brain stimulation patients. As such, this report has been acknowledged to be exempt from formal IRB review by our institutional IRB. These patients underwent unilateral or bilateral VIN–thalamic DBS implantation by a single surgeon at an academic medical center between March 2004 and January 2011. Five out of eight of the patients underwent DBS programming by a practitioner at the medical center conducting the current analysis of the clinical data set and the remaining three patients were programmed by an outside neurologist. All patients had severe tremor preoperatively, and all patients had failed to achieve adequate benefit from at least one anti–tremor medication. All patients carried a diagnosis of multiple sclerosis confirmed by a neurologist not involved with the current analysis. Patients were evaluated at 3–5 weeks postoperatively, and programming was started at or immediately following this visit. Patients presented again at 2–3 months postoperatively, and tremor control continued to be described by the examiner at this visit, as well as throughout the follow–up period, wherein standard practice is to evaluate patients annually.
Specific outcome measures were collected both pre and postoperatively, and throughout the follow–up period. Tremor control was determined by subjective patient and family care–giver report, as well as from relevant clinical notes. Neuropsychological data was collected at baseline before the surgery and then at repeated intervals, usually 3 and 6 months postoperatively and then annually at the anniversary of surgery. At our institution, neuropsychological evaluations are a standard clinical evaluation for patients who are considering undergoing DBS, and are an essential component of the regular follow–up regimen. Such practice allows us to relate any potential changes in neurocognitive function to immediately preoperative measurements.
Three patients who underwent DBS placement before 2008 did not undergo adequate neuropsychological testing during the follow–up period, so data for these patients was limited to subjective patient reports and examiner reports of tremor control following implantation, at first follow–up after programming, and at last available follow–up. The remaining five patients were analyzed with respect to response of tremor, as well as with respect to changes in neuropsychological measures at last available followup when compared to those same measures during the preoperative evaluation period. Neuropsychological outcome measures included the controlled oral word association test (COWAT), the hopkins verbal learning test (HVLT), and the beck depression inventory–II (BDI–II). Three of the five patients also had a documented pre and postoperative Mini–mini-mental state exam (MMSE).
Results
A series of eight consecutive patients (6 women, 2 men) who underwent VIN–thalamic DBS implantation for medication–resistant MS tremor were reviewed. Four patients underwent bilateral VINthalamic implantation, three underwent unilateral VIN placement, and one underwent right VIN placement followed 3 months later by left VIN placement. The mean patient age at implantation was 45 years (range 38–49 years). The mean number of years between diagnosis of MS and DBS implantation was 10.6 years (range 2–21 years). The mean duration of total postoperative follow–up was 30.5 months (range 14– 71 months), and the mean duration of follow–up for the subset of five patients who underwent complete neuropsychological testing was 19 months (range 14–25 months).
All patients were evaluated with regards to tremor control throughout the follow–up period. Evaluation consisted of both patient and examiner report of tremor control. Two of the eight patients experienced microlesion effect that was still present at the first postoperative appointment, before programming was started. The remaining six patients were at their baseline in terms of tremor, by both patient and examiner report, at the first postoperative follow–up. Only four out of the eight patients reported improvement in tremor control at the second appointment, the first appointment after programming was started. One of these four included one of the patients who had experienced a microlesion effect. Of the remaining four patients who did not report improvement at the second appointment, one had experienced a microlesion effect, underwent programming, then experienced return of tremors, was reprogrammed, and finally experienced improvement of tremors, noted at the next follow–up appointment. A second patient denied improvement after initial programming and the examiner was equivocal; however the patient’s father and caregiver both reported improvement in the patient’s ability to perform activities of daily living (ADLs). Programming was modified, and the patient experienced clear improvement in tremor by the next appointment. A third patient denied improvement in tremor after initial programming but reported improvement in ADLs. This patient was also reprogrammed, and also experienced improvement in tremor by the next follow–up appointment. The final of the four patients who did not experience improvement in tremor after initial programming was reprogrammed and experienced complete relief of tremor at last follow–up. All eight patients had some improvement of tremor at the last available follow–up with the device turned on. One patient had improvement in tremor at the penultimate followup appointment, but then had the device turned off due to concern for seizure activity, and had no tremor control at the last follow–up. Seizure activity was not felt by the neurologist or the neurosurgeon to be related to the DBS, but the Emergency Department had instructed the patient to deactivate the device.
All five patients who underwent DBS implantation after 2008 were evaluated both pre and postoperatively with neuropsychological testing. Neuropsychological outcome measures included the COWAT, the HVLT and the BDI–II. Three of the five patients also had a documented pre and postoperative MMSE.
The MMSE is one of the simplest and best known screening tests for neurocognitive impairment and was documented at both pre and post–operative states for three patients. The first patient scored 30⁄30 on both visits. The second patient scored 24⁄27 preoperatively and 20⁄27 postoperatively. A total score of 27 was used due to visual impairment. Lastly, the third patient scored 27⁄30 both pre– and postoperatively.
COWAT is a test of verbal fluency and is sensitive to frontal impairment. The following scores were adjusted for patient age, education and gender, per published normative data. The scores are presented as percentiles. A test of letter fluency was administered. Review of the results of the COWAT revealed that the first patient scored in the 40th percentile both pre and postoperatively. The second patient scored in the 16th percentile preoperatively, and scored 19th postoperatively. The third patient scored in the 35th percentile preoperatively and 26th percentile postoperatively. The fourth patient scored in the 39th percentile preoperatively, and in the 37th percentile postoperatively. The final patient scored in the 26th percentile preoperatively, but scored in the 17th percentile postoperatively. When comparing the pre and postoperative scores, patients lost an average of 3.4 percentile points during follow–up (range –9 to +3 percentile).
The HVLT is a word–list learning and memory task which consists of both immediate and delayed recall. The immediate recall is a measure of the ability to learn while the delayed recall is a measure of the ability to remember. The first patient scored 26⁄36 for the immediate recall preoperatively and 27⁄36 for the immediate recall postoperatively. The same patient scored 10⁄12 for the delayed recall preoperatively and 9⁄12 postoperatively. The second patient scored 12⁄36 and 0⁄12 preoperatively, dropping to 5⁄36 and 0⁄12 postoperatively. The third patient scored 25⁄36 and 7⁄12 preoperatively, and 20⁄36 and 8⁄12 postoperatively. The fourth patient scored 19⁄36 and 9⁄12 preoperatively, and 16⁄36 and 7⁄12 postoperatively. Finally, the fifth patient scored 12⁄36 and 0⁄12 preoperatively, and 16⁄36 and 1⁄12 postoperatively.
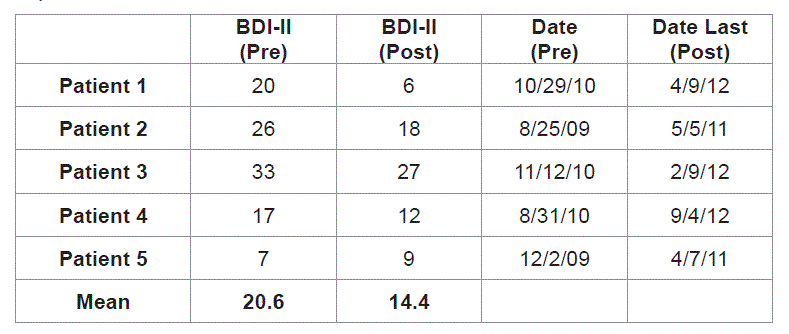
The BDI–II is a well–established clinically–relevant tool to assess depression with a lower numerical score correlating with a less depressed state. This data was reviewed for all five patients. The first patient had a preoperative BDI–II of 20, which fell to a postoperative BDI–II of 6. The second patient decreased from 26 preoperatively to 18 postoperatively. The third patient decreased from 33 to 27, and the fourth patient decreased from 17 to 12. The fifth patient was the only patient to demonstrate an increase in BDI–II, from 7 preoperatively to 9 postoperatively, but both of these scores are in the non–depressed range and therefore this increase was not clinically significant. The average decrease in BDI–II score for all patients was 6.2 points (range = gain of 2 points to loss of 14 points). Thus most patients were less depressed or showed no change after the intervention.
The results of all neuropsychological testing are summarized in Table 1, Table 2 and Figure 1.
COWAT
(Pre)
COWAT
(Post)
HVLT-immediate
(Pre)
HVLT-
Immediate
(Post)
HVLT-
Delayed
(Pre)
HVLT-
Delayed
(Post)
MMSE
(Pre)
MMSE
(Post)
Patient 1
37
37
26
27
10
9
30/30
30/30
Patient 2
13
15
12
5
0
0
26/27*
20/27*
Patient 3
35
26
25
20
7
8
-
26/30
Patient 4
39
37
19
16
9
7
27/30
27/30
Patient 5
26
17
12
16
0
1
-
-
Mean
30
26.4
18.8
16.8
5.2
5
20.6
14.4
Table 1: Results of neuropsychological testing.
*Note: A total score of 27 was used for Patient 2 due to visual impairment (items for reading, writing, and drawing were eliminated). The maximum score for each tests are as follows: COWAT (100th percentile), HVLT immediate [36], HVLT delayed [12], BDI–II [63], MMSE [30]
Neuropsychiatric
Test
Results
Implications
COWAT
No clinically significant change
Evaluates the spontaneous production of word beginning with a given letter or belonging to a given category within a limited amount of time. Tests verbal fluency and executive function with high sensitivity for detecting frontal impairment. DBS unlikely to influence these parameters.
HVLT
(immediate, delayed)
No clinically significant change
Tests word-list learning and memory task which consists of both the immediate and delayed recall. The immediate recall is a measure of the ability to learn while the delayed recall is a measure of the ability to remember. DBS unlikely to influence these parameters.
BDI-II
No significant change with trend toward improved mood
Well-establish clinically-relevant tool to assess depression with a lower numerical score correlating with a less depressed state. DBS unlikely to worsen depression with possibility of improving mood state.
Table 2: Summary and implications of the results derived from the neuropsychological tests.
Figure 1: Average changes of neuropsychiatric data. There were no clinically significant changes, indicating neurocognitive decline was not observed following DBS. It should again be noted that a smaller value on the BDI-II testspecifies a less depressed state, suggesting that patients on average showed an improvement in mood.
Discussion
Numerous studies have demonstrated the effectiveness of VINthalamic DBS in ameliorating the cerebellar outflow tremor of MS, albeit with varying methods and length of follow–up. Notably, Aziz, demonstrated that tremor reduction can be permanent for up to a mean of 5.2 years [12]. By presenting positive outcome measures in eight consecutive patients documented over a mean of 30.5 months, with one case benefiting up to 71 months, our data adds support to the hypothesis that DBS is an effective therapy to achieve long term control of tremor seen in MS patients. Our analysis also highlights an advantage of DBS over thalamotomy noted by Yap [9] by documenting reversibility and adjustability following the initial programming in order to obtain subjective tremor control. Importantly, none of our patients suffered major neurologic complications, with one patient demonstrating seizure activity not attributable to the DBS. Thus, it can be inferred that VIN–thalamic DBS is likely a safe and effective procedure for subjective long term tremor reduction in MS patients.
Prior to sensitive neurocognitive testing, the prevalence of cognitive difficulties was grossly underestimated to affect less than 5% of MS patients. Over the last 25 years, studies have clearly shown that as many as 40–60% of patients suffer from cognitive problems [8]. It is critical, therefore, to be cognizant of subtle neuropsychiatric complications following treatment of MS symptoms. From our data, we could infer that, in general, DBS does not appear to cause worsening of neuropsychological outcomes. None of our patients suffered deterioration in terms of verbal fluency (COWALT), learning and memory (HVLT) and depression (BDI–II) that was felt to be clinically significant by the evaluating neuropsychologist. The possible improvement of depression observed in our patients is potentially an important finding and demands further study since suicidal ideation and risk of self–harm is higher than expected in those with MS [13]. Admittedly, this increase could be due to chance alone as no statistical analysis was performed, given the absence of a control or comparison group.
One potential limitation to our analysis involves the lack of objective measure of tremor severity. We assessed tremor improvement by documenting subjective reports provided by patients as well as their caregivers and healthcare providers. Although not as uniform and well–defined, personal feeling of symptom improvement better represents WHO’s definition of health by focusing on improving the ‘complete physical, mental, and social well–being and not merely the absence of disease or infirmity.’ Also, the natural progression and complex integration of varying neurological deficits seen in this patient population make post–surgical objective measures difficult [5] and frankly meaningless unless associated with subjective symptomatic relief. It is critical to recall that tremor is associated with the natural progression of the disease and does not currently have any truly effective medical alternative for treatment. If the goal is to improve the quality of life, any proposed tremor treatment needs to provide symptomatic benefit appreciated by the patient and⁄or caregiver without adding unnecessary burden.
Another limitation of this review is that parallel neuropsychological testing was not conducted in a control group of similar MS patients. Furthermore, the clinical significance of any observed change in neuropsychological outcomes was defined subjectively by the Neuropsychologist involved with the study. However, it is important to note that a review of the literature demonstrates an overall decline in neuropsychological outcomes with time in the MS population. For example, a study assessing the effect of ginkgo biloba on cognitive function in MS patients included a control group that received placebo alone, and this group demonstrated a decline in COWAT over time [14]. Thus it is possible that DBS does not alter the baseline progression of neuropsychological outcomes in MS patients.
Furthermore, this study involved a total of eight patients, only five of whom underwent neuropsychological testing. The small number of patients is a limitation of the current study, and a larger study would be warranted.
Finally, most patients underwent programming by their neurologist, not all of whom were employed by the academic medical center performing the DBS surgery. For this reason, stimulation parameters, such as frequency, voltage, amplitude, and pulse width, were not available for all patients. Further work could potentially detail stimulation parameters in association with tremor control outcomes.
With such potential to improve tremor and decrease disability without causing significant neuropsychological complications, DBS may be an important adjunct to the overall therapy for MS related tremor. Future research should focus on selecting the best patient population that will benefit from this intervention. Some suggestions include elucidating the proper timing of surgery relative to the natural progression of the disease, determining which sub–type of MS will derive the greatest benefit, and assessing whether any coinciding symptoms beside tremors are associated with eventual well–being or complications following DBS. Each study should strive to include a large patient sample with a comparable control group. They should be adequately randomized and gather both subjective and objective measures of tremor severity as well as pre and postoperative neuropsychological assessment including changes in mood.
Conclusion
Our data adds support to the theory that DBS is an effective therapy to achieve long term control of tremor in MS patients. We also demonstrated the advantages of DBS over thalamotomy by documenting reversibility and adjustability following the initial operation. Once again, none of our patients suffered major neurologic complications. It can therefore be inferred that VIN–thalamic DBS is likely a safe and effective procedure for long term tremor reduction in MS patients.
We could also infer from our results that DBS does not appear to cause worsening of neuropsychological outcomes. None of our patients suffered clinically significant deterioration in terms of verbal fluency, learning and memory, and depression. Of note, the possible improvement of depression observed in our patients is an important finding and demands further study since suicidal ideation and risk of self–harm is higher than expected in those with MS.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this analysis or the findings specified in this paper.
References
- Noonan CW, Kathman SJ, White MC . Prevalence estimates for MS in the United States and evidence of an increasing trend for women. Neurology.2002; 58: 136-138.
- Bradley WG, Daroff RB, Fenichel GM, Jankovic J. Bradley’s Neurology in Clinical Practice. 2008; 5: 1583-1621.
- Torres CV, Moro E, Lopez-Rios AL, Hodaie M, Chen R, Laxton AW, et al. Deep brain stimulation of the ventral intermediate nucleus of the thalamus for tremor in patients with multiple sclerosis. Neurosurgery.2010; 67: 646-651.
- Loonstra AS, Tarlow AR, Sellers AH. COWAT metanorms across age, education, and gender.ApplNeuropsychol. 2001; 8: 161-166.
- Hosseini H, Mandat T, Waubant E, Agid Y, Lubetzki C, Lyon-Caen O, et al. Unilateral thalamic deep brain stimulation for disabling kinetic tremor in multiple sclerosis. Neurosurgery.2012; 70: 66-69.
- Patten SB .Treatment of neuropsychiatric syndromes in multiple sclerosis. Expert Rev Neurother. 2005; 5: 413-420.
- Arnett PA, StroberLB . Cognitive and neurobehavioral features in multiple sclerosis. Expert Rev Neurother. 2011; 11: 411-424.
- Brassington JC, Marsh NV .Neuropsychological aspects of multiple sclerosis.Neuropsychol Rev. 1998; 8: 43-77.
- Yap L, Kouyialis A, VarmaTR . Stereotactic neurosurgery for disabling tremor in multiple sclerosis: thalamotomy or deep brain stimulation?. Br J Neurosurg.2007; 21: 349-354.
- Schuurman PR, Bruins J, Merkus MP, Bosch DA, SpeelmanJD .A comparison of neuropsychological effects of thalamotomy and thalamic stimulation.Neurology.2002; 59: 1232-1239.
- Bittar RG, Hyam J, Nandi D, Wang S, Liu X, Joint C, et al. Thalamotomy versus thalamic stimulation for multiple sclerosis tremor. J ClinNeurosci.2005; 12: 638-642.
- Thevathasan W, Schweder P, Joint C, Ray N, Pretorius P, Gregory R, et al. Permanent tremor reduction during thalamic stimulation in multiple sclerosis. J NeurolNeurosurg Psychiatry.2011; 82: 419-422.
- Pompili M, Forte A, Palermo M, Stefani H, Lamis DA, Serafini G, et al. Suicide risk in multiple sclerosis: a systematic review of current literature. J Psychosom Res. 2012; 73: 411-417.
- Lovera JF, Kim E, Heriza E, Fitzpatrick M, Hunziker J, Turner AP, et al. Ginkgo biloba does not improve cognitive function in MS: a randomized placebo-controlled trial. Neurology.2012; 79: 1278-1284.