Review Article
Austin J Clin Neurol 2014;1(3): 1012.
Spontaneous Cervical Artery Dissections
Steinsiepe VK, Jung S, Goeggel Simonetti B and Arnold M*
Department of Neurology, Bern University Hospital, Switzerland
*Corresponding author: Marcel Arnold, Department of Neurology, Bern University Hospital, Freiburgstrasse 10, 3010 Bern, Switzerland
Received: March 19, 2014; Accepted: June 11, 2014; Published: June 15, 2014
Abstract
Spontaneous cervical artery dissection is a rare cause of stroke in general, but it is a major cause of stroke in young adults. While connective tissue abnormalities and minor trauma may be involved, to this date, its pathogenesis remains unclear. Genetic mutations may also play a role and a seasonal pattern as well as associations with migraine, high plasma homocysteine and chiropractic manipulative therapy have been found. Clinical presentation comprises of local and, more importantly, ischemic symptoms, but only recently randomized controlled trials have been started to assess thrombolysis and antithrombotic treatment. Because early diagnosis could possibly prevent or alleviate ischemic manifestations, these patients should be assessed quickly and accurately. The following review aims to provide a well-rounded insight into the disease.
Keywords: Cervical artery dissection; Spontaneous; VAD
Abbreviations
CAD: Cervical Artery Dissection; ICAD: Internal Carotid Artery Dissection; VAD: Vertebral Artery Dissection
Introduction
First pathological reports of cervical artery dissection (CAD) date back as far as 1872, but the condition was only recognized as a cause of stroke as late as the mid-1950s [1]. While at first considered a rather rare disease [2], CAD has gained a lot of attention over the past decades and is now recognized as one of the most important etiologies of stroke in young people [3], accounting for more than 2% of strokes in the general population and about 20% in adults under the age of 45 [2,4,5], therefore being an equally frequent cause of stroke as cardiac embolism [3].
Pathology
CAD is defined by the presence of an intramural hematoma in a cervical artery, mostly the internal carotid artery or the vertebral artery. The relatively high frequency of dissections in cervical arteries compared to dissections in other arteries of the same diameter maybe partially explained by their greater mobility. This led to the recognition of CAD as a more or less independent pathology [2].
Two kinds of pathomechanisms have been postulated to lead to CAD: Either, for unknown reasons, the tunica intima tears apart, and blood enters through the tear and forms a false lumen within the tunica media with the subsequent development of an intramural hematoma [2]. Or, after initial rupture of the vasa vasorum, the primary formation of an intramural hematoma causes the intimal tear [6].
The dissection itself usually appears distally to the tear [5,7]. A subintimal entry of blood is thought to lead to primary dilatation of the vessel wall into the original vessel lumen causing stenosis or occlusion while a sub-adventitial hemorrhage would primarily dilate outwardly and form a so-called dissecting aneurysm [2,8]. While these aneurysms sometimes have been referred to as pseudoaneurysms or false aneurysms, these terms should not be used because a pseudoaneurysm is not contained by the arterial wall, but by the surrounding tissue, in contrast to the dissecting aneurysm, the boundaries of which always partially include the blood vessel [2,8].
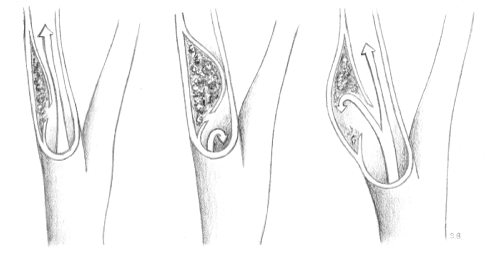
Cervical artery dissections are divided into three subtypes (Figure 1): They may present as stenotic in about half of all cases and occlusive as well as of dissecting aneurysmal form in about one-fourth each, but numbers may vary and combined forms occur [8-10]. The higher the degree of stenosis, the more likely are ischemic events, while aneurysmal forms are thought to cause more local symptoms [10,11]. Aneurysmal dilatation occurs usually in the sub-petrous segment of the internal carotid artery [10]. Aneurysms are described as either saccular (pouchy) or fusiform (dilating) [8].
Figure 1: Stenotic, occlusive and aneurysmal cervical artery dissection.
In almost all cases, the affected vessels are the internal carotid and the vertebral artery. Internal carotid artery dissection (ICAD) is usually restricted to the cervical portion of the artery, most cases starting from about 2cm above the carotid bifurcation and not exceeding its entry into the petrous part of the temporal bone. Vertebral artery dissection (VAD) usually occurs proximal to the dural part and mostly in the pars transversaria (V2) and the atlas loop (V3), however all segments can be affected [12]. It has been noted that ICAD is generally located at the transitory region between elastic and muscular artery type and ontogenetic factors might therefore be involved in its development [13].
Cervical artery dissections have also been subcategorized into spontaneous, iatrogenic and traumatic, the latter owing to a higher incidence after major trauma to the neck as for example in motor vehicle accidents [14]. Major trauma may be the cause of dissection in up to one fourth of cases [15]. But while in these settings the connection is compelling, in most cases patient history only includes trivial or no trauma at all and the nearly endless list of everyday activities associated with trivial trauma (ranging from playing golf over sneezing to prolonged rotation of the head during a phone call) and the disease would necessarily lead to an incidence much higher than so far reported, eliminating the possibility of trivial trauma being the sole cause [16]. Spontaneous cervical artery dissections therefore include cases in which minor injury has occurred [17].
Epidemiology
In one French and two American studies, the annual incidence of spontaneous CAD has been observed to be about 2.6 to 2.9 per 100 000 population, being higher in urban than in rural areas [9,18,19]. The separate incidence for spontaneous internal carotid artery dissection and spontaneous vertebral artery dissection is about 1.7 and 1 per 100 000, respectively [9]. Increased awareness in clinicians and the availability of noninvasive diagnostic tools may have raised recognition of CAD [20]. However, these numbers have been stated to be an underestimation because spontaneous dissection can be asymptomatic, which is known from incidental findings of CAD [2].
Patients presenting with spontaneous CAD have been reported to be male in about 57% and female in 43% [21,22]. The mean patient’s age is about 45 years, but men are approximately 5 years older at the time dissection occurs [21,22]. VAD occurs about 3 years earlier than ICAD [22]. A male predominance has been observed especially in children (<18) and could not be explained by a higher rate of preceding trauma or physical activity [23].
Between 4 and 40% of CADs extend intracranially [5]. Such extensions are much more frequent in VAD [5]. Intracranial cases are often associated with younger age as well as with dissecting aneurysms, subarachnoidal hemorrhage, more severe infarcts and subsequently a substantially poorer outcome [5,24]. In at least 15%of patients, more in women, less in men, multiple dissections are found [5,21,22].
Pathogenesis
After almost two generations of research, the causes of spontaneous CAD are still not resolved in detail [6,25,26]. An underlying arteriopathy has been suggested from the very beginning but has not been identified so far [6]. In the same manner, the presence of a mechanical factor has been emphasized [27]. Research nowadays is focused on treatment efficacy and on the identification of risk factors. Both suffer from the rather poor level of evidence, which in turn is a consequence of the rather low incidence of the disease, the statistical comparison with stroke-from-other-cause patients instead of healthy controls, several forms of bias and conflicting evidence (see below) [25].
Nevertheless, numerous possible risk factors have been identified, discussed and divided into two major groups: constitutional and environmental [25].
Constitutional risk factors
Constitutional risk factors mostly comprise of conditions attributable to a single or numerous abnormalities of the vascular system. More recently, the search for genetic predispositions has been intensified [28]. The origin of this suspicion lies within early reports of a relatively high number of patients with genetic disorders often affecting the connective tissue and ultrastructural connective tissue abnormalities that have been found in about half to two-thirds of spontaneous CAD patients, leading towards an affected biosynthesis of extracellular matrix [26,29,30]. Alterations have also been found in the families of patients, but only in less than 5% of cases CAD patients are related to one another [29].
Together with the plausibility of an underlying structural defect of the arterial wall, this has led to the search of an underlying hereditary connective tissue disease, which could only be identified in about 1-5% of all cases [2]. These hereditary connective tissue diseases comprise mainly of Ehlers-Danlos syndrome type IV, Marfan’s syndrome, Loeys-Dietz syndrome, autosomal polycystic kidney disease, cystic medial necrosis and osteogenesis imperfecta type I [2,24,26]. Other disorders were discovered in these patients, foremost fibromuscular dysplasia in about 10-15% [2,5,15].
The claim of an underlying arteriopathy has been further supported by a high incidence of vascular abnormalities, such as increased arterial distensibility, arterial redundancies and intracranial aneurysms [2]. Importantly, CAD is considered to be distinct from atherosclerotic disease [29].
The continuous search for risk factors and potential triggering events now spreads, among others, over low levels of alpha1- antitrypsin, high plasma homocysteine, inflammatory mechanisms, migraine, gene polymorphisms and mutations and combinations of any of those, such as the recent promising candidate gene MTHFR/ C677T, which has been linked to hyperhomocysteinemia [17,25,26].
Noteworthy, migraine was found to be associated with a doubled risk of CAD [31]. This may be plausible, as migraine has been linked to the vascular system as well as to stroke before [31].
In any case, with for example the MTHFR-gene being supported by one meta-analysis but contradicted by a second one, substantial findings have not exactly been made [25,26,32].
Environmental risk factors
A frequently mentioned environmental risk factor is trivial trauma [2,6]. In many patients, a history of minor trauma is provided, but in most cases the temporal link remains the only proven one [6]. In only 18% of histopathologic studies traces of mechanical damage could be found [13]. The role of trivial trauma is probably overestimated and has been called not an “important and frequent” risk factor for CAD [29].
Chiropractic manipulative therapy (CMT) is highly disputed and has been linked to CAD, especially VAD, in some 6% of cases [6]. Theoretically, the stretching of the vertebral artery during rotation or the fixation of the internal carotid artery against the vertebrae during rotation combined with flexion would be held accountable [14,33], but the forces applied have been measured to not be high enough to damage the vessel [7]. There is a possibility of confounding bias for the temporal association between dissection and chiropractor visit, arguing that CMT might be frequented because of neck pain due to dissection or an underlying cause of dissection [7,14,33], as well as a considerably high chance of selection bias [14].
Other environmental risk factors include recent infection, this claim being upheld by the finding of a seasonal pattern of CAD with most cases occurring in autumn [29]. Also, smoking and contraceptive use have been discussed, but not been proven [4].
Despite promising results of the structural analysis of connective tissue of patients, research of possible risk factors has so far not achieved any major breakthroughs, with different reviewers reaching the conclusion that supporting evidence is sparse and Rubinstein et al. stating that “in fact, some of the supposed risk factors may even be protective” [25,26]. Spontaneous dissection may likely be the result of various combinations of genetic predisposition and constitutional and environmental factors [21,29].
Clinical Presentation
Symptoms of CAD are generally considered to be local or ischemic. While stroke is the most feared complication, almost 80% of patients present themselves at first with other symptoms, often leaving sufficient time in which stroke could possibly be prevented [15]. However, prevention remains challenging with the time window ranging from minutes to hours in about half of patients [15]. The typical clinical manifestations are listed in Tables 1 and 2.
Internal carotid artery
Vertebral artery
Headache (frontotemporal) and/or neck pain (anterior)
Horner’s syndrome
Pulsatile tinnitus
Cranial nerve palsies (mainly IX-XII)
Headache (occipital) and/or neck pain (posterior)
Cervical radiculopathy
Table 1: Local clinical findings in patients with cervical artery dissection, ordered by frequency.
Internal carotid artery
Vertebral artery
Ischemic stroke
TIA
Transient monocular blindness
Retinal infarct
Ischemic stroke
TIA
Cervical spinal ischemia
Table 2: Ischemic presentation of patients with cervical artery dissection, ordered by frequency.
Local symptoms
The local impairment of the surrounding structures could be either of mechanical origin or due to an affected blood supply [13]. Headache and neck pain are the most common finding and are experienced in up to 78% of patients with CAD [34]. They often precede cerebral ischemia and are therefore not only considered to be an important first clue, but also a differential feature to atherosclerotic disease [17]. While these two symptoms are the most common presentation, asymptomatic forms are possible, although rare [2].
Local symptoms: ICAD
For ICAD, a classic triad has been described, constituting of the presence of unilateral pain of the anterior head, face or neck and an ocular sympathetic palsy also called partial Horner’s syndrome later followed by retinal or cerebral ischemia. Because only one third of patients present with the complete triad, the attending clinician should suspect ICAD even if only two of these symptoms are present [2].
Two-thirds of patients are experiencing a mostly ipsilateral, frontal or frontotemporal headache [34]. In about half of patients, pain is described in the facial or orbital region and little over one fourth of patients suffer from neck pain [34]. Only in about 10% of cases, pain is the only presenting symptom [34]. The quality of the headache is constant steady aching and usually its onset is gradual, but other courses are not uncommon [34]. The median time between pain and the development of further symptoms is about 4 days, ranging from seconds to weeks [34].
Horner’s syndrome, mostly without facial anhydrosis, is another typical finding, present in less than 50% of patients [34]. The absence of facial anhydrosis is explained by the facial sweat glands being innervated by the sympathetic plexus, which surrounds the external and not the internal carotid artery [2].
Further local symptoms of ICAD are pulsatile tinnitus sometimes accompanied by bruit on auscultation in about 25% and cranial nerve palsy in about 12%. They are generally affecting the lower cranial nerves [2]. Painful Horner’s syndrome and cranial nerve palsy are thought to be more common in patients with dissecting aneurysms [10].
Local symptoms: VAD
The clinical presentation of VAD has been described as being difficult to distinguish from other differential diagnoses [34]. While also presenting with pain, this finding seems to be more prone to the back of the head and neck. It may be misinterpreted as being of musculoskeletal origin [2].
Headache is more common than neck pain (about two-thirds vs. half) and tends to affect only one occipital side, with a throbbing or steady quality and usually a gradual onset [17,34]. At the same time, neck pain is more common in patients with VAD than with ICAD [34]. The median time from headache to onset of other symptoms is about 14.5 hours [34].
Local symptoms may also affect the cervical roots, causing radicular syndromes [2].
Ischemic symptoms
Unfortunately, cerebral infarction is not only the most feared, but also the most common complication of CAD, affecting about two-thirds of patients [12,15,21]. The mechanism of infarction could either be of primary hemodynamic origin due to the stenosis, or secondary due to embolism [11]. The detection of emboli by transcranial Doppler and infarction patterns correspond with an embolic cause in the vast majority of patients [35-37].
Another possible complication is subarachnoid hemorrhage, which more often occurs in VAD than in ICAD and in about 3% of CADs [11]. With more than 20%, the rate is much higher in intracranial dissections [36].
Ischemic symptoms: ICAD
The rate of stroke in ICAD is higher than 50% and in up to 80% it is preceded by local symptoms, a transient ischemic attack (TIA) or amaurosis fugax [15,17,38]. Ischemia generally manifests itself in the middle cerebral artery territory. In earlier times, the assumed rate of stroke was higher than today; it has dropped because through the development of diagnostic procedures, more cases with less severe manifestations can be detected [2,38].
Ischemic symptoms: VAD
Stroke is more common in VAD than in ICAD, occurring in about 80%, and nearly all patients are affected by either cerebral infarction or TIA [17,34]. The ischemia usually affects the posterior circulation and may lead to Wallenberg’s syndrome, gait ataxia, partial Horner’s syndrome or visual field abnormalities [2,12,17,39]. Subarachnoidal hemorrhage occurs in about 5%, but the rate rises up to 23% in patients with intracranial VAD [17]. The intracranial vessels are more prone to rupture, because they contain less elastic tissue and are thinner [17]. Because of the artery's rather unrestricted entrance into the skull, about 10% of VADs extend intracranially [5].
Clinical Presentation in Children
For unknown reasons the vast majority of children present with cerebral ischemia and most commonly hemiparesis [23]. In this age group, pain is not the leading symptom, with headache in only about half of patients and neck pain being a rarity [23]. It has been suggested, that this may reflect the failure to diagnose children in an earlier phase of CAD [23]. Furthermore, spontaneous dissection of the anterior circulation but not of the posterior circulation is more likely to be intra- than extracranial in children, suggesting a different pathophysiology [23].
Diagnosis
The diagnostics of CAD rely on imaging techniques [4]. Conventional Angiography, the former gold standard, has been replaced by magnetic resonance imaging and magnetic resonance angiography [2]. Other frequently used tools are CT angiography and cervical ultrasound [40].
Ultrasound
As a first diagnostic procedure, ultrasonographic techniques are useful because one can detect abnormal flow patterns in about 90% of patients [2]. However, diagnostic abnormalities of CAD, such as an echogenic intimal flap or a floating thrombus, are only found in less than one-third and ultrasound may be normal in about 30% of patients presenting with local symptoms only [2,17,41].
Conventional Angiography (CA)
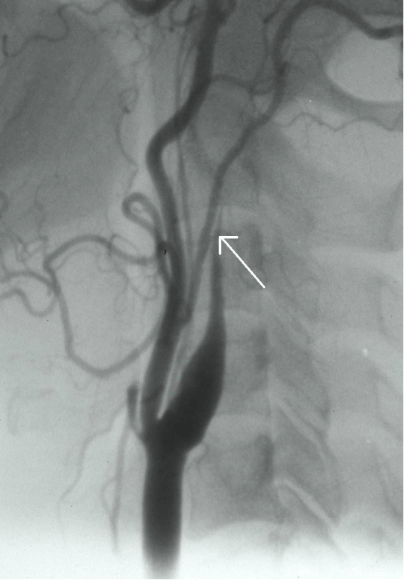
Luminal abnormalities seen with conventional angiography may feature various unspecific findings such as stenosis of variable length with string-sign or gradual narrowing with flame-like occlusion (Figure 2).Pathognomonic signs such as a double-lumen or an intimal flap are rare [2,42].
Figure 2: Digital substraction catheter angiography showing a typical flame shaped occlusion of the carotid artery in a patient with internal carotid artery dissection.
CT Angiography (CTA)
Compared with MRI / MRA, the test characteristics of CT / CTA are very similar, but to date clinical experience is limited [20]. CTA has the potential to show the early hematoma and in the traumatic setting, CTA is more feasible [20].
MR Angiography (MRA) and MR Imaging (MRI)
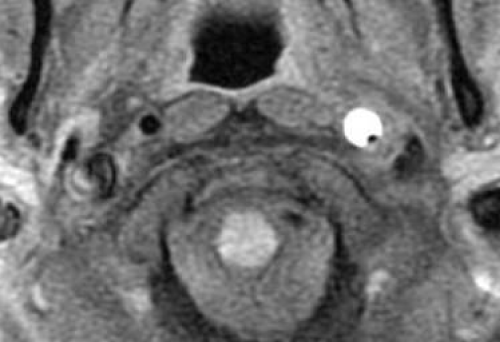
The main reason for MRI / MRA being the new gold standard is its noninvasiveness. Additionally, MRA can depict the intramural hematoma itself and therefore does not or cannot respectively rely on the luminal abnormalities, which are the basis of CA [2]. The vast majority of diagnoses in the past decade have been made by MRI / MRA, but usually in combination with other procedures. The hematoma presents itself as a hyperintense area beside the vessel lumen (Figure 3) and may spiral along its length [2,17]. Taking DSA as a reference, MR techniques have a high sensitivity and specificity, approximately 83 and 99% respectively, but studies on these specifications have delivered widely varying results [20]. The main disadvantage of MRI is that in the acute stage, the hematoma may be isointense, making the detection of CAD rather difficult [17]. MR techniques are also less sensitive for VAD because of the artery’s smaller diameter and anatomic variability [20,17]. However, newer generations of MRI continue to improve its diagnostic yield.
Figure 3: MRI with T1 fat suppression technique showing a mural hematoma in the left internal carotid artery in a patient with carotid artery dissection.
Treatment
The treatment of CADs relies on the experience of treatment of stroke of other causes. There has not been any completed randomized controlled trial on any treatment of CADs yet and there is only one ongoing trial [1,43].
Acute treatment in CAD patients with stroke contains three possible alternatives. Thrombolysis is the most widely advocated one, endovascular treatment has recently emerged as relatively safe and surgery is generally considered to be a last resort [44]. Secondary prevention is achieved by the use of antithrombotics.
Thrombolysis
Thrombolysis can be applied intravenously, intra-arterially, or both. In the vast majority of cases, intravenous thrombolysis is used [40]. The theoretical concerns with thrombolysis are a possible enlargement of the intramural bleeding causing the stenosis to grow, promoting hemodynamic ischemia, dislocation of the thrombus, embolic infarcts and facilitating formation of dissecting aneurysms and subarachnoid hemorrhage [40,45-47]. However, it may in turn recanalize the vessel [40].
Randomized controlled trials are not available [1], but relatively large studies have been conducted [40,46]. Thrombolyzed CAD patients have worse outcome than thrombolyzed patients with stroke of other cause [40].
The theoretical complications of thrombolysis include symptomatic intracranial hemorrhage [40,46]. This occurs in about 5% of CAD, but this rate appears not to be increased by thrombolysis [47]. In any case, it is considered to be a low complication rate and thrombolysis in acute ischemic stroke is believed to be relatively safe in CAD patients [45,47].
Endovascular Procedures
Endovascular treatment is usually considered when medical management fails or is contraindicated [43]. Usually, a metallic stent, ideally a self-expanding one, is applied to reopen a stenosis, to seal the wall of the vessel or to occlude a dissecting aneurysm [43,45]. This procedure is generally followed by a combination of aspirin and clopidogrel [43]. Other endovascular treatment options include direct thrombus aspiration and injection of thrombolytic agents into the hematoma [48].
About half of dissection-related endovascular procedures are used to treat traumatic dissections, despite the fact that spontaneous dissections are more common. The technical success rate is high, lying just below 100%, and the perioperative complications are few, ranging around 1% [43,45,48]. In-stent stenosis has been reported in varying numbers, ranging from 2% to almost half of cases, and the overall stroke rate has been reported to be 11% in one systematic review [43,48].
Stenting necessity and relative efficacy are disputable, because stenotic lesions tend to reopen spontaneously in the majority of cases and risk of embolization from dissecting aneurysms has been reported to be low [8,45]. It has also been stated that there is insufficient evidence to draw definite conclusions about thrombolysis and stenting in dissection [45], but the lack of evidence towards any benefit of intravenous thrombolysis has prompted Qureshi et al. [46] to propose the evaluation of additional endovascular procedure following thrombolysis in CAD patients with moderate to severe strokes. However, the long-term outcome of carotid stenting is unknown [44].
Surgery
Surgical treatment consists of ligation, resection or bypassing of the vessel with for example a saphenous vein graft [13,44]. However, because the complication rate is high (mortality 2-11%, minor recurrent stroke in 10%, lower cranial nerve damage in 58% causing mostly temporary dysphagia, dysphonia and hoarseness), surgery has for the most part been replaced by endovascular treatment [1,2,13].
Antithrombotics
The question, whether anticoagulation or antiplatelets as antithrombotic treatment are superior or inferior to one another, has been widely discussed [1,11,44].
Anticoagulation usually consists of initial i.v. heparin, followed by oral warfarin, aiming for an INR from 2 to 3 for 3 to 6 months. This approach has been advocated since the 1970s and is based on the mostly thromboembolic origin of stroke in CAD [35-37]. Antiplatelets comprise mainly of acetylsalicylic acid and clopidogrel [44].
Similarly to thrombosis, theoretical concerns state that anticoagulation may enlarge the intramural hematoma with subsequent worsening of local symptoms and increase of stenosis [45,49]. The association of higher frequencies of delayed internal carotid artery occlusions with higher degrees of anticoagulation supports this claim, however in most patients this is not associated with clinical worsening [11].
On the other hand, recent reviews concluded that this effect has not been shown and no evidence exists promoting the use of either anticoagulation or antiplatelets [1,45,49]. No significant difference could be shown neither for the prevention of recurrent stroke, nor the outcome of death and disability nor the occurrence of bleeding complications [1,49]. Because anticoagulation does not seem to be more effective than antiplatelets in the prevention of recurrent strokes but on the other hand has more potential complications, the Cochrane meta-analysis concluded: “[…] patients with extracranial internal carotid artery dissection are unlikely to be harmed by antiplatelet drugs and there seems little justification for giving anticoagulants as a first line therapy in all patients”[1]. This seems to be attributable to vertebral artery dissections as well [49]. Other authors have recommended deciding whether to choose antiplatelets or anticoagulants on a case by case basis considering clinical presentation, vessel status and potential bleeding risk [50].
Out of fear of a potentially higher frequency of subarachnoid hemorrhages, anticoagulation has never been recommended for patients with an intracranial extension of the dissection [2,11]. Indeed intracranial bleeding complications are more frequent in intracranial CAD, but their incidence is still very low [17].
Prognosis
The prognosis of CAD depends on the severity of the ischemic event and the grade of stenosis [2,15]. The risk of stroke is highest within the first few days [46]. The outcome tends to be better for spontaneous than for traumatic ICAD with between 75 and 92% recovering well, meaning a score of 0-2 on the modified Rankin Scale [9,17,32]. About 12% are severely disabled or dead [15]. VAD has been associated with an excellent outcome in thrombolyzed patients and an overall better outcome and a lower rate of stroke than ICAD [42,47]. About 10% of treated patients experience headaches for more than a week, sometimes up to many years [2]. After stroke, the rate of favorable outcome declines to little over 50% [40].
From an anatomic point of view, outcome is generally favorable, because 90% of stenosis resolve, up to two-thirds of occlusions reopen and one third of dissecting aneurysms decrease in size. These changes usually happen within 3 months [2]. VADs recanalize completely more often than ICADs and vertebral dissecting aneurysms resolve more often than carotid ones [10,42].
Mortality is probably less than 5% [5,46]. With about 11%, mortality is much higher in children [23]. While mortality in adults is rather low, bad outcome (mRS > 2) is still to be expected in about 20% [21], and although CAD patients tend to be young, their outcome after stroke is generally worse than in patients with stroke from other causes [46].
Recurrence
A distinction between the rate of recurrent dissection and of recurrent stroke is made in the literature. Only rarely symptoms such as TIA are included, but one prospective study reported an annual rate of all recurrent events of 10.4% [39].
Recurrent dissection
The rate of recurrent dissection is 2-3.2% in the first month and then 0.3-1.6% per year, with an overall rate between 4% and 12%, and has therefore been described as uncommon, but not rare [5,9,15,27]. Interestingly, recurrent dissection rarely occurs within the originally affected vessel [5,27].
Recurrent stroke
There have been differing reports about the rate of recurrent stroke in CAD patients. Numbers range from less than 1% to 4% and medical therapy does not seem to influence this rate substantially [1,11,49]. In most studies, the early rate of recurrent stroke in the first month is around 2% per month, and it decreases to about 0.5% or less per year, most events therefore occurring late and recurrence being rare [42,51,52]. This rate is only insignificantly higher in patients with severe stenosis or occlusion [51]. It is however higher in patients with multiple dissections [52].
Extracranial dissecting aneurysms and recurrence
There have been concerns that the aneurysmal forms may be prone to either thrombi formation and embolism or rupture, giving rise to discussions about their management [8]. Unlike stenotic and occlusive dissections, 32-65% of extracranial dissecting aneurysms remain anatomically unchanged over time. No influence of the aneurysm type on the outcome has been observed [10,37].
No correlation between emboli (monitored by transcranial Doppler) or stroke and the presence of dissecting aneurysms could be found [37] and summarizing 3 long-term follow-up studies of extracranial aneurysmal ICADs no patient out of a total of 89 had any recurrent event over mean time periods of 6.5, 3 and 3.5 years respectively [8,10,53]. Moreover, no case of a ruptured extracranial dissecting aneurysm has been published in the literature [2,8]. The relatively good outcome of this type of dissection supports a conservative treatment strategy in the vast majority of patients because the complications of a more aggressive approach potentially outweigh its benefits [8,10,54].
Conclusions
Half a century after recognizing CAD as a cause of stroke, most of the origins and mechanisms of this disease still lie in the dark. The link to connective tissue anomalies and diseases has been made but the underlying and triggering factors remain unclear. More pathologic research is needed, especially regarding the promising results of a very small number of studies in this field. Since most patients fortunately survive the disease, a multicenter, collaborative approach seems appropriate.
On the clinical side, several ongoing multicenter studies have the potential to give new insights into the role of genetics and environmental risk factors, most notably trauma and infection [28]. Additionally, the introduction of MRI/MRA as a diagnostic standard and its ability to picture the intramural hematoma will hopefully fuel progress and lead to more accurate diagnosis of the disease.
Treatment is mainly based on pathophysiological considerations, observational studies and analogy to treatment of stroke of other causes. In terms of antithrombotic treatment, clinicians will have to make a decision between acetylsalicylic acid and oral anticoagulants based on pathophysiology, clinical evaluation and imaging findings until the results of ongoing and future studies are fully available.
In summary, little is known and much remains to be discovered in the field of cervical artery dissections. Large prospective multicenter studies are needed to further elucidate etiology, pathogenesis, diagnosis and treatment. The sparse knowledge of this disease does not reflect its importance: About one-fifth of strokes in young adults are caused by cervical artery dissection [2].
Acknowledgement
We are grateful to Sandra Burren, BMed, for the depiction of cervical artery dissections in Figure 1.
References
- Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. 2010; CD000255.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001; 344: 898-906.
- Nedeltchev K, der Maur TA, Georgiadis D, Arnold M, Caso V, Mattle HP, et al. Ischaemic stroke in young adults: predictors of outcome and recurrence. J Neurol Neurosurg Psychiatry. 2005; 76: 191-195.
- Blunt SB, Galton C. Cervical carotid or vertebral artery dissection. BMJ. 1997; 314: 243.
- Schievink WI, Mokri B, O'Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994; 330: 393-397.
- Haneline MT, Lewkovich GN. An analysis of the etiology of cervical artery dissections: 1994 to 2003. J Manipulative Physiol Ther. 2005; 28: 617-622.
- Miley ML, Wellik KE, Wingerchuk DM, Demaerschalk BM. Does cervical manipulative therapy cause vertebral artery dissection and stroke? Neurologist. 2008; 14: 66-73.
- Guillon B, Brunereau L, Biousse V, Djouhri H, Lévy C, Bousser MG, et al. Long-term follow-up of aneurysms developed during extracranial internal carotid artery dissection. Neurology. 1999; 53: 117-122.
- Lee VH, Brown RD Jr, Mandrekar JN, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006; 67: 1809-1812.
- Touzé E, Randoux B, Méary E, Arquizan C, Meder JF, Mas JL, et al. Aneurysmal forms of cervical artery dissection : associated factors and outcome. Stroke. 2001; 32: 418-423.
- Engelter ST, Brandt T, Debette S, Caso V, Lichy C, Pezzini A. Antiplatelets versus anticoagulation in cervical artery dissection. Stroke. 2007; 38: 2605-2611.
- Arnold M, Bousser MG, Fahrni G, Fischer U, Georgiadis D, Gandjour J, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006; 37: 2499-2503.
- Müller BT, Luther B, Hort W, Neumann-Haefelin T, Aulich A, Sandmann W, et al. Surgical treatment of 50 carotid dissections: indications and results. J Vasc Surg. 2000; 31: 980-988.
- Haneline M, Triano J. Cervical artery dissection. A comparison of highly dynamic mechanisms: manipulation versus motor vehicle collision. J Manipulative Physiol Ther. 2005; 28: 57-63.
- Dziewas R, Konrad C, Dräger B, Evers S, Besselmann M, Lüdemann P, et al. Cervical artery dissection--clinical features, risk factors, therapy and outcome in 126 patients. J Neurol. 2003; 250: 1179-1184.
- Haldeman S, Kohlbeck FJ, McGregor M. Risk factors and precipitating neck movements causing vertebrobasilar artery dissection after cervical trauma and spinal manipulation. Spine (Phila Pa 1976). 1999; 24: 785-794.
- Kim YK, Schulman S. Cervical artery dissection: pathology, epidemiology and management. Thromb Res. 2009; 123: 810-821.
- Schievink WI, Mokri B, Whisnant JP. Internal carotid artery dissection in a community. Rochester, Minnesota, 1987-1992. Stroke. 1993; 24: 1678-1680.
- Giroud M, Fayolle H, André N, Dumas R, Becker F, Martin D, et al. Incidence of internal carotid artery dissection in the community of Dijon. J Neurol Neurosurg Psychiatry. 1994; 57: 1443.
- Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009; 193: 1167-1174.
- Metso AJ, Metso TM, Debette S, Dallongeville J, Lyrer PA, Pezzini A, et al. Gender and cervical artery dissection. Eur J Neurol. 2012; 19: 594-602.
- Arnold M, Kappeler L, Georgiadis D, Berthet K, Keserue B, Bousser MG, et al. Gender differences in spontaneous cervical artery dissection. Neurology. 2006; 67: 1050-1052.
- Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children. Neurology. 2001; 57: 1155-1160.
- Pelkonen O, Tikkakoski T, Leinonen S, Pyhtinen J, Sotaniemi K. Intracranial arterial dissection. Neuroradiology. 1998; 40: 442-447.
- Rubinstein SM, Peerdeman SM, van Tulder MW, Riphagen I, Haldeman S. A systematic review of the risk factors for cervical artery dissection. Stroke. 2005; 36: 1575-1580.
- Debette S, Markus HS. The genetics of cervical artery dissection: a systematic review. Stroke. 2009; 40: e459-466.
- Bassetti C, Carruzzo A, Sturzenegger M, Tuncdogan E. Recurrence of cervical artery dissection. A prospective study of 81 patients. Stroke. 1996; 27: 1804-1807.
- Debette S, Metso TM, Pezzini A, Engelter ST, Leys D, Lyrer P, et al. CADISP-genetics: an International project searching for genetic risk factors of cervical artery dissections. Int J Stroke. 2009; 4: 224-230.
- Brandt T, Grond-Ginsbach C. Spontaneous cervical artery dissection: from risk factors toward pathogenesis. Stroke. 2002; 33: 657-658.
- Hausser I, Müller U, Engelter S, Lyrer P, Pezzini A, Padovani A, et al. Different types of connective tissue alterations associated with cervical artery dissections. Acta Neuropathol. 2004; 107: 509-514.
- Rist PM, Diener HC, Kurth T, Schürks M. Migraine, migraine aura, and cervical artery dissection: a systematic review and meta-analysis. Cephalalgia. 2011; 31: 886-896.
- McColgan P, Sharma P. The genetics of carotid dissection: meta-analysis of a MTHFR/C677T common molecular variant. Cerebrovasc Dis. 2008; 25: 561-565.
- Rothwell DM, Bondy SJ, Williams JI. Chiropractic manipulation and stroke: a population-based case-control study. Stroke. 2001; 32: 1054-1060.
- Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995; 45: 1517-1522.
- Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998; 29: 2646-2648.
- Pelkonen O, Tikkakoski T, Pyhtinen J, Sotaniemi K. Cerebral CT and MRI findings in cervicocephalic artery dissection. Acta Radiol. 2004; 45: 259-265.
- Srinivasan J, Newell DW, Sturzenegger M, Mayberg MR, Winn HR. Transcranial Doppler in the evaluation of internal carotid artery dissection. Stroke. 1996; 27: 1226-1230.
- Biousse V, D'Anglejan-Chatillon J, Touboul PJ, Amarenco P, Bousser MG. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. 1995; 26: 235-239.
- Beletsky V, Nadareishvili Z, Lynch J, Shuaib A, Woolfenden A, Norris JW; Canadian Stroke Consortium. Cervical arterial dissection: time for a therapeutic trial? Stroke. 2003; 34: 2856-2860.
- Engelter ST, Dallongeville J, Kloss M, Metso TM, Leys D, Brandt T, et al. Thrombolysis in cervical artery dissection--data from the Cervical Artery Dissection and Ischaemic Stroke Patients (CADISP) database. Eur J Neurol. 2012; 19: 1199-1206.
- Baumgartner RW, Arnold M, Baumgartner I, Mosso M, Gönner F, Studer A, et al. Carotid dissection with and without ischemic events: local symptoms and cerebral artery findings. Neurology. 2001; 57: 827-832.
- Arauz A, Hoyos L, Espinoza C, Cantú C, Barinagarrementeria F, Román G. Dissection of cervical arteries: Long-term follow-up study of 130 consecutive cases. Cerebrovasc Dis. 2006; 22: 150-154.
- Pham MH, Rahme RJ, Arnaout O, Hurley MC, Bernstein RA, Batjer HH, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 2011; 68: 856-866.
- Schievink WI. The treatment of spontaneous carotid and vertebral artery dissections. Curr Opin Cardiol. 2000; 15: 316-321.
- Menon R, Kerry S, Norris JW, Markus HS. Treatment of cervical artery dissection: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2008; 79: 1122-1127.
- Qureshi AI, Chaudhry SA, Hassan AE, Zacharatos H, Rodriguez GJ, Suri MF, et al. Thrombolytic treatment of patients with acute ischemic stroke related to underlying arterial dissection in the United States. Arch Neurol. 2011; 68: 1536-1542.
- Zinkstok SM, Vergouwen MD, Engelter ST, Lyrer PA, Bonati LH, Arnold M, et al. Safety and functional outcome of thrombolysis in dissection-related ischemic stroke: a meta-analysis of individual patient data. Stroke. 2011; 42: 2515-2520.
- Donas KP, Mayer D, Guber I, Baumgartner R, Genoni M, Lachat M. Endovascular repair of extracranial carotid artery dissection: current status and level of evidence. J Vasc Interv Radiol. 2008; 19: 1693-1698.
- Kennedy F, Lanfranconi S, Hicks C, Reid J, Gompertz P, Price C, et al. Antiplatelets vs anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology. 2012; 79: 686-689.
- Arnold M, Fischer U, Bousser MG. Treatment issues in spontaneous cervicocephalic artery dissections. Int J Stroke. 2011; 6: 213-218.
- Kremer C, Mosso M, Georgiadis D, Stöckli E, Benninger D, Arnold M. Carotid dissection with permanent and transient occlusion or severe stenosis: Long-term outcome. Neurology. 2003; 60: 271-275.
- Touzé E, Gauvrit JY, Moulin T, Meder JF, Bracard S, Mas JL; Multicenter Survey on Natural History of Cervical Artery Dissection. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology. 2003; 61: 1347-1351.
- Benninger DH, Gandjour J, Georgiadis D, Stöckli E, Arnold M, Baumgartner RW, et al. Benign long-term outcome of conservatively treated cervical aneurysms due to carotid dissection. Neurology. 2007; 69: 486-487.
- Liberati A, Altmann DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339: b2700.