Research Article
Austin J Clin Neurol 2014;1(3): 1013.
Immunological and Endocrine Profile of Two Patients with Severe Subarachnoid Neurocysticercosis, Resistant to the Cysticidal Treatment
Laura Adalid-Peralta1,2, Gladis Fragoso3, Edda Sciutto3, Marisela Hernández3, Agnès Fleury2,3, Helgi Jung1 s and Graciela Cárdenas1*
National Institute of Neurology and Neurosurgery, Mexico
Peripheral Unit of the Institute for Biomedical Research at the National Institute of Neurology and Neurosurgery, México
Institute of Biomedical Research, National Autonomous University of Mexico; Mexico
*Corresponding author: Graciela Cárdenas, National Institute of Neurology and Neurosurgery, D.F., 14269, Mexico City, Mexico
Received: February 21, 2014; Accepted: July 21, 2014; Published: Aug 04, 2014
Abstract
Neurocysticercosis (NC) is a disease caused by the establishment of Taenia solium larvae in the central nervous system. NC may exhibit different clinical pictures depending on the location of the established cysticerci, their degenerating stage, and the intensity of the immune-inflammatory profile induced by the infection. Moreover, the response to cysticidal treatment is also heterogeneous. When cysticerci establish in the subarachnoid space of the base (SABNC) they are more frequently resistant to the treatment.
To further deepen our understanding of this resistant status, the central and peripheral inflammatory and endocrine profiles of two SABNC patients who failed to respond to four treatment cycles (albendazole plus corticosteroids) are herein reported.
Several immunological and endocrinological treats were found altered: decreased levels of testosterone, cortisol, and prolactin were observed, accompanied by increased levels of immunomodulatory cytokines (TGF-β and IL-10), as well as LH.
Altogether, these results establish the impact of SABNC on the immune-inflammatory and endocrine status of both patients. The possible relevance of these changes in the NC pathogenesis is discussed.
Keywords: Taenia solium; Neurocysticercosis; Treatment resistance; Taeniasolium
Abbreviations
NC: Neurocysticercosis; SABNC: Subarachnoid of the base Neurocysticercosis; CSF: Cerebrospinal Fluid; INNN: Instituto Nacional de Neurología y Neurocirugía; ABZ: Albendazole; ABZ-SO: Albendazole Sulfoxide; HPLC: High-performance Liquid Chromatography; CT: Computed tomography; MRI: Magnetic resonance imaging; DXM: Dexamethasone; PZQ: Praziquantel; MTX: Methotrexate; PDN: Prednisone
Background
Human cysticercosis is a parasitic infection caused by the establishment of Taenia solium larvae (cysticerci) in human tissues. In most cases, the parasite establishes in muscles and in the brain. The latter location leads to neurocysticercosis (NC), the most severe form of the disease [1]. The clinical presentation of NC varies from asymptomatic to mild to a severe neurological disease with intracranial hypertension. This heterogeneity mainly depends on the parasite location in the central nervous system [2]. NC patients lodging cysticerci in the parenchyma generally show an inflammatory response restricted to the area surrounding the cysts, exhibit a non-inflammatory cerebral spinal fluid (CSF) [2], and an effective response to the cysticidal treatment is usually observed [3]. In contrast, when cysticerci are located in the ventricles or in the subarachnoid space of the base (SABNC), most cases are accompanied by an increased CSF cellularity and may result in secondary intracranial hypertension. Several findings support the hypothesis that the parasite gained, thorough millions of years of co-evolution with the host, the ability to manipulate its environment, promoting a comfortable milieu that favor its survival [4,5]. This is seems to be a common strategy developed by some large parasites that may persist for years in the host tissues without apparent inflammatory response [6].
The endocrine system could also participate in this immunomodulation, as it has been reported in other host-parasite interactions, both under experimental and natural conditions [7]. Regarding human NC few information and the role of immunoendocrine system is available [8].
In this report, we present the inflammatory and endocrine profiles of two SABNC patients harboring multiple parasites and who did not respond to several cycles of conventional cysticidal treatment (non-responder patients).
Case Presentations
Case 1
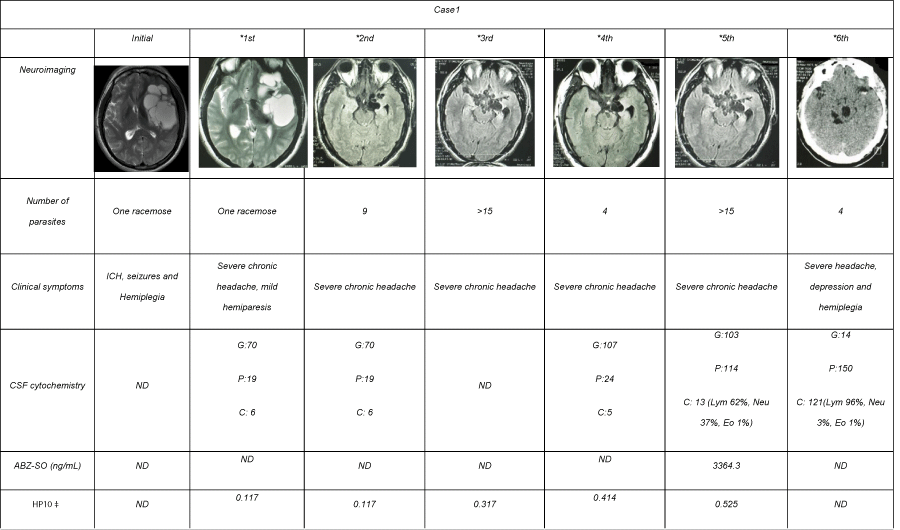
A 29 year-old Mexican man living in the United States of America suffered from sudden generalized seizures. CT scan showed NC. The patient was administered ABZ plus steroids. Afterward, progressive motor dysphasia, right sided weakness, and intracranial hypertension signs appeared and the patient came back to Mexico. MRI showed extensive racemose cysticerci on left Sylvian fissure with displacement of anatomical structures off the medial line. ABZ at 30 mg/kg, plus dexamethasone (DXM) at 0.3 mg/kg were administered during 8 days. Motor weakness improved in the following months, and a control MRI showed a 95%-reduction of the giant racemose cysticercus, along with asymmetric dilation of the left ventricle secondary to encephalomalacia (the side where parasites were previously lodged), and also multiple new parasitic lesions in the cerebral media artery cisterns. Clinical improvement continued until 4 months later, when the patient developed suddenly intense headache associated to visual blurring and diplopia. The patient was hospitalized to receive an additional ABZ cycle with close medical supervision. In the clinical and radiological follow-up at 7, 9, 11, and 19 months, basal parasites located in the perimesencephalic region persisted and a new parasite in the third ventricle appeared. A total of 6 ABZ and DXM cycles and two combined ABZ and praziquantel (PZQ) cycles were administered, with neither clinical nor radiological evidence of parasite elimination (Table 1). (Some data from this patient were partially reported in BMC Neurol 2010:10:16.) [9].
Table 1: Clinical and radiological follow-up of case1 during ABZ cycles.
Case 2
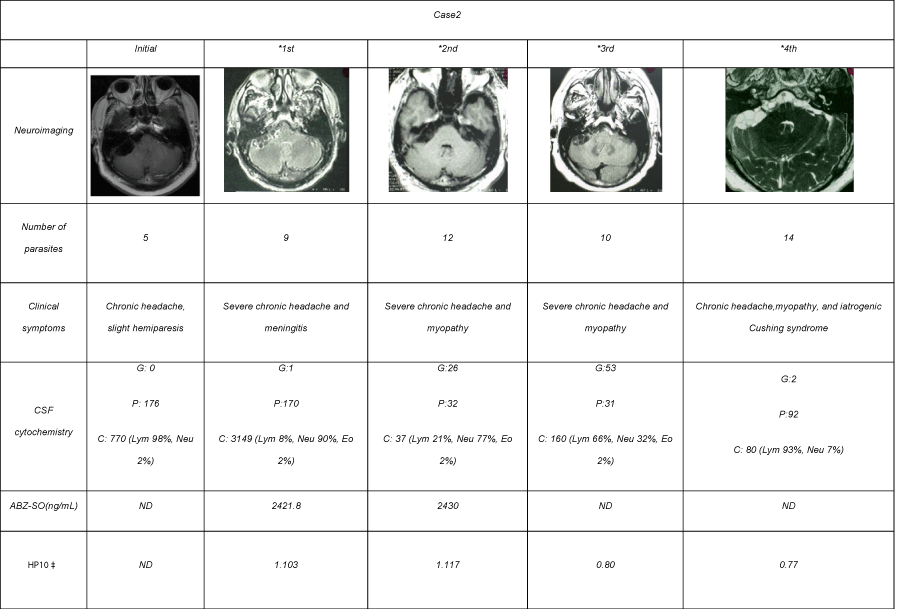
A 60 year-old Mexican man with a history of chronic headache. Nine months before hospital admission, headache frequency increased and it was associated with lower limb motor dysfunction. Neurological examination upon admission revealed only papilledema. On MRI, subarachnoid NC in the posterior fossa was observed and the patient received the first course of ABZ at 30 mg/kg plus DXM 0.3 mg/kg by 8 days. Mild clinical improvement was observed, but four months later the previous neurological alterations worsened. Due to adverse effect of steroid administration, methotrexate (MTX) was added instead of steroids. No radiological changes in the parasite number or size were observed on MRI, and a second course of ABZ and DXM at the same doses was administered. In the next monthly follow-ups, MRI showed parasite persistence, and a combined course of two cysticidal drugs (ABZ at 30 mg/kg and PZQ at 50 mg/kg) associated to DXM at 0.3 mg/kg was administered by 8 days. CSF analysis showed an inflammatory profile, with abundant lymphocytes and eosinophils, in spite of prednisone (PDN) and MTX administration. No radiological disappearance of parasites was observed in a control MRI, and the patient remained symptomatic after 3 cycles of ABZ alone and one combined cycle of ABZ and PZQ (Table 2).
Table 2: Clinical and radiological follow-up of case 2 during ABZ cycles.
Material and Methods
mmune-endocrine profiling and CSF analysis were conducted during the clinical follow-up of patients. The clinical and radiological characteristics of both patients are summarized in Tables 1 & 2. Several analyses on CSF recovered by lumbar puncture were conducted during clinical follow-up; these data are summarized in Table 3. A control group of 5 neurological patients with epilepsy or idiopathic intracranial hypertension and without NC was included.
Ethical considerations
This study fulfills all research regulations for human beings required by Mexican laws and international regulations. Patients volunteered to enter the study, donated blood and CSF samples, and signed an informed consent form.
Biological specimens
Blood and CSF samples were aseptically taken from both patients during clinical follow-up and frozen at –80°C in sterile containers until used.
Investigations
Cytokine levels
CSF cytokine levels in NC patients were measured using a Th1/Th2 Cytokine Kit II (BD eBiosciences Pharmingen) and a cytometric bead array. The procedure was carried out according to the manufacturer’s directions. Fluorescence measurement and analysis were performed using a FACSCalibur flow cytometer and the BD Cytometric Bead Array software (BD Biosciences Pharmingen). Flow cytometer was calibrated by using BD FACSComp (BD Biosciences Pharmingen) and BD CaliBRITE Beads (BD Biosciences Pharmingen). Assay sensitivities were as follows: IL-6, 3.0pg/mL; IL-10, 2.8pg/mL; TNF-α, 2.8pg/mL, and IFN-γ, 7.1pg/mL. TGFβ (sensitivity: 9.4 pg/mL) was measured by using the human/mouse TGF-beta 1 ELISA Ready-SET-Go (Bioscience, San Diego, CA). ELISA was performed according to the manufacturer’s instructions. All samples were run in duplicate.
HP10 antigen detection
To evaluate the parasite viability after NC treatment, HP10 antigen was detected by Ag-ELISA as previously described [8]. All samples were run in duplicate. Briefly, Immulon I plates (Nunc, Rochester, New York, USA) were coated with monoclonal HP10 antibody (100mL at 10mg/mL in 0.07 M NaCl buffered with 0.1 M borate, pH 8.2) and left overnight at 4°C. Plates were washed four times with 200mL/well of wash solution (0.9% w/v NaCl containing 0.05% v/v Tween 20). Plates were blocked using 200mL diluents (phosphate-buffered saline containing bovine serum albumin 1.0% w/v and 0.05% v/v Tween 20) and left for 60 min at room temperature before being washed in a similar way. Undiluted CSF or serum samples (100mL/well) were added and incubated for 30 min at 37°C. Bound HP10 parasite antigen was detected using biotinylated monoclonal antibody HP10 (1:4000 in diluents, for 30 min at 37°C), horseradish peroxidase-conjugated streptavidin (Zymed, San Francisco, California, USA) (1:4000 in diluents, 30 min at 37oC) and tetramethylbenzidine (Zymed) as substrate. The color reaction was allowed to proceed for 30 min at 37°C in the dark and was stopped by adding 100mL 0.2 M H2SO4 (Baker, Estado de México, Mexico). Optical density (450 nm) was determined in an ELISA processor (Versamax microplate reader, Molecular Devisable, Sunnyvale, California, USA).
Plasmatic albendazole sulfoxide levels
Both patients showed hepatic and renal function in normal ranges. 4 mL-blood and CSF samples were collected at the seventh day of treatment, 8 hours after the last albendazole (ABZ) dose. Albendazole sulfoxide (ABZ-SO) was measured by HPLC and expressed in ng/mL.
During hospitalization, ABZ was administered at 30 mg/ kg (ZENTEL, tablets 200 mg, GlaxoSmithKline) along with dexamethasone sodium phosphate at 0.3 mg/kg/d.
Hormone profile
Shortly after blood collection, serum was separated and kept at 207d C until required. The concentration of the following hormones was measured by radioimmunoanalysis (RIA) using 125I tracer kits: testosterone (TESTO-CT2), luteinizing hormone (LH) (RIA-gnost hLH), prolactin (RIA-gnost PROL), and cortisol (CORT-CT2), all from CIS-bio international (Gif sur Yvette, France). Detection limits were: cortisol, 4.6nmol/L; testosterone, 0.1nmol/L; LH, 0.15mUI/mL, and prolactin, 5μU/mL.
Results
CSF cytochemistry
A long-term clinical follow-up was conducted in both SABNC patients. As shown in Table 1, CSF from patient 1 showed little inflammatory traits until the fifth ABZ cycle. After the sixth cycle (20 months after treatment onset), the inflammatory reaction increased, with pleocytosis of 121 cells/mm3 associated with hypoglycorrachia. In patient 2, an inflammatory profile was observed since the first lumbar puncture with 773cells/mm3, increasing to 3149 cells/mm3 after the first ABZ cycle, and with concomitant clinical manifestations of meningeal syndrome. Persistence of pleocytosis and hypoglycorrachia were observed in spite of intensive anti-inflammatory treatment.
Plasmatic levels of ABZ sulfoxide
ABZ sulfoxide levels were measured to evaluate whether the minimum therapeutic level of 1700 ng/mL proposed by Góngora- Rivera et al [10] as necessary to destroy cysticerci was reached in the central nervous system. As Table 1 shows, the concentration found in patient 1 after the fifth treatment cycle (3364.3 ng/mL), and the level in patient 2 after the first (2430 ng/mL) and second cycle (2421.8 ng/ mL), were both above the minimum required.
CSF cytokine levels
Patient 1 showed high CSF-IFN levels before the first ABZ cycle and also high IL10 levels before the fifth ABZ cycle. Increased plasmatic TGF-β levels were found before each ABZ cycle. TNF-α level were irregular, but a 2-fold increase was found from the first to the fourth ABZ cycle.
Hormone profile
Plasmatic testosterone and cortisol levels were decreased, and LH levels were increased (Table 3). Patient 2 showed low IFN-γ levels and high IL-6 and IL-10 levels in CSF before treatment. Plasmatic testosterone, as well as prolactin and cortisol levels, were decreased, and LH levels were increased (Table 2). The positive HP10 optical density in both patients confirmed the parasite viability throughout the clinical follow-up (Tables 1 & 2).
ABZ cycles
TGFß
(pg/mL)
IFN-γ
(pg/mL)
TNF-α
(pg/mL)
IL-10
(pg/mL)
IL-6
(pg/mL)
LH
(mUI/mL)**
Testosterone (nmol/L)**
Prolactin
(μU/mL)**
Cortisol
(nmol/L)**
C 1Δ
1st
1312.5
7.1
20
2.8
ND
3.66
3.9
131.5
0.5
3rd
4200
0
0
2.8
ND
2.7
3.3
ND
0.6
4th
2275
0
48.6
2.8
ND
ND
ND
ND
ND
5th
1093.95
0
0
14.9
ND
ND
ND
ND
ND
C 2▲
1st
ND
0
4.9
140.5
5000
3.3
3.3
6.4
5.4
3rd
ND
ND
ND
ND
ND
3.8
4.9
13.1
8.2
4th
ND
ND
ND
ND
ND
ND
4.5
7.8
10.2
Controls
46 ±26**
>9
6.2 ±2.1
9.4± 1.2
4.7± 0.8
1.9 ±0.85
21.93±2.8
20.9 ± 0.25
30.5 ± 0.13
Table 3: Immunoendocrinological profile of the two SABNC cases during ABZ cycles.
Renal and liver function tests were normal during all clinical follow-up.
Discussions and Conclusions
This study describes the clinical, immunological, and endocrine profiles of two SABNC patients with severe clinical manifestations, both of whom failed to respond to cysticidal treatment after several administration cycles with adequate albendazole sulfoxide levels. Persistent inflammatory characteristics were observed in CSF (hypoglycorrachia, hyperproteinorrachia, and pleocytosis). The secreted HP10 antigen levels found in both patients’ CSF and MRI results confirmed that cysticerci remained alive during all the evaluation period.
While we are aware of the reported differences between lumbar and ventricular CSF traits for inflammatory process during NC [11], in our study we analyzed lumbar CSF compartment and we found that the inflammatory prolife (glucose, proteins and cell number) in both patients was accompanied with changes in cytokine levels that reinforce the possibility that an inflammatory process is occurring in the CNS.
A decrease in prolactin levels was observed in patient B, while in both patients a sustained decrease in testosterone and cortisol levels was observed. Both NC patients exhibited an important reduction in testosterone levels with respect to those expected in the normal population and in our controls, a finding that points to possible endocrinological alterations in these patients, these finding have been previously reported findings in severe NC patients, like the two patients herein described [8]. Testosterone was also found decreased in naturally infected, cysticercotic boars in comparison with non-cysticercotic animals [12]. This has also been observed in mice chronically infected with Taenia crassiceps cysticerci. In these mice, testosterone is progressively reduced until the observed sex-hormone profile resembles that of female mice [13]. With regard to the relation between reduced testosterone levels and the infection, testosterone can affect directly the parasite growth. In vivo, the parasite could use the host steroid precursors for its own development, leading to a testosterone decrease during cysticercosis in humans, boars, and mice [8,14,15]. This testosterone consumption may result in its depletion, and it is a permissive factor for cysticercus development.
The mechanisms that mediate the neuroimmunoendocrine environment in the host merit some comments [6, 16-19]. Different modulating mechanisms have been reported in related parasites. Schistosome-specific phosphatidylserine (PS) activates TLR and affects dendritic cells; as a result, mature dendritic cells gained the ability to induce IL-10-producing regulatory T cells [20]. Also, excretory/secretory products of Echinococcus multilocularis larvae induce in vitro tolerogenic properties in dendritic cells [21]. These findings make conceivable that cysticercus-secreted molecules may promote a regulatory immune environment that may hamper the host response to the cysticidal treatment. Recently, it has been reported that viable cysticerci induce regulatory T cells [22], using dendritic cells as intermediary [23]. Therefore, it is possible that parasites could favor a regulatory environment to survive in an immunocompetent host, in spite of treatment.
With regard to the complex interactions between hormones and the immune system, it has been extensively reported that the incidence and severity of natural parasitic infections show a sexual dimorphism that involves a distinct exposure in males and females to various parasite infective stages; likewise, differential effects of sex steroids on immune cells and direct effects of these steroids on parasites, among other effects, have also been reported. Typically, the prevalence and intensity of a large number of parasitic diseases are higher in males than in females. However, in several parasitic infections males are more resistant than females [24].
Taken together, these data pointed out to a possible deep impact of severe cysticercal infection on the immune-inflammatory and endocrine status of NC patients.
Acknowledgement
Authors thank Juan Francisco Rodriguez for proofreading the English version of this manuscript. This study was supported by CONACYT (CB-2008-01 100708, CB-2011-01 167278) and by DGAPA IN213911.
References
- Sciutto E, Fragoso G, Fleury A, Laclette JP, Sotelo J, Aluja A, et al. Taenia solium disease in humans and pigs: an ancient parasitosis disease rooted in developing countries and emerging as a major health problem of global dimensions. See comment in PubMed Commons below Microbes Infect. 2000; 2: 1875-1890.
- Alvarez JI, Colegial CH, Castaño CA, Trujillo J, Teale JM, Restrepo BI, et al. The human nervous tissue in proximity to granulomatous lesions induced by Taenia solium metacestodes displays an active response. See comment in PubMed Commons below J Neuroimmunol. 2002; 127: 139-144.
- Monteiro L, Almeida-Pinto J, Stocker A, Sampaio-Silva M. Active neurocysticercosis, parenchymal and extraparenchymal: a study of 38 patients. See comment in PubMed Commons below J Neurol. 1993; 241: 15-21.
- Fleury A, Dessein A, Preux PM, Dumas M, Tapia G, Larralde C, et al. Symptomatic human neurocysticercosis--age, sex and exposure factors relating with disease heterogeneity. See comment in PubMed Commons below J Neurol. 2004; 251: 830-837.
- Hoberg EP, Alkire NL, de Queiroz A, Jones A. Out of Africa: origins of the Taenia tapeworms in humans. See comment in PubMed Commons below Proc Biol Sci. 2001; 268: 781-787.
- Escobedo G, López-Griego L, Morales-Montor J. Neuroimmunoendocrine modulation in the host by helminth parasites: a novel form of host-parasite coevolution? See comment in PubMed Commons below Neuroimmunomodulation. 2009; 16: 78-87.
- Bottasso O, Morales-Montor J. Neuroimmunomodulation during infectious diseases: mechanisms, causes and consequences for the host. See comment in PubMed Commons below Neuroimmunomodulation. 2009; 16: 65-67.
- Cárdenas G, Valdez R, Sáenz B, Bottasso O, Fragoso G, Sciutto E, et al. Impact of Taenia solium neurocysticercosis upon endocrine status and its relation with immuno-inflammatory parameters. See comment in PubMed Commons below Int J Parasitol. 2012; 42: 171-176.
- Cárdenas G, Carrillo-Mezo R, Jung H, Sciutto E, Hernandez JL, Fleury A, et al. Subarachnoidal Neurocysticercosis non-responsive to cysticidal drugs: a case series. See comment in PubMed Commons below BMC Neurol. 2010; 10: 16.
- Göngora-Rivera F, Soto-Hernández JL, González Esquivel D, Cook HJ, Márquez-Caraveo C, Hernández Dávila R, et al. Albendazole trial at 15 or 30 mg/kg/day for subarachnoid and intraventricular cysticercosis. See comment in PubMed Commons below Neurology. 2006; 66: 436-438.
- Torres-Corzo JG, Tapia-Pérez JH, Sánchez-Aguilar M, Della Vecchia RR, Chalita Williams JC, Cerda-Gutiérrez R, et al. Comparison of cerebrospinal fluid obtained by ventricular endoscopy and by lumbar puncture in patients with hydrocephalus secondary to neurocysticercosis. See comment in PubMed Commons below Surg Neurol. 2009; 71: 376-379.
- Peña N, Morales J, Morales-Montor J, Vargas-Villavicencio A, Fleury A, Zarco L, et al. Impact of naturally acquired Taenia solium cysticercosis on the hormonal levels of free ranging boars. See comment in PubMed Commons below Vet Parasitol. 2007; 149: 134-137.
- Morales-Montor J, Escobedo G, Vargas-Villavicencio JA, Larralde C. The neuroimmunoendocrine network in the complex host-parasite relationship during murine cysticercosis. See comment in PubMed Commons below Curr Top Med Chem. 2008; 8: 400-407.
- Larralde C, Morales J, Terrazas I, Govezensky T, Romano MC. Sex hormone changes induced by the parasite lead to feminization of the male host in murine Taenia crassiceps cysticercosis. See comment in PubMed Commons below J Steroid Biochem Mol Biol. 1995; 52: 575-580.
- Huerta L, Terrazas LI, Sciutto E, Larralde C. Immunological mediation of gonadal effects on experimental murine cysticercosis caused by Taenia crassiceps metacestodes. See comment in PubMed Commons below J Parasitol. 1992; 78: 471-476.
- Escobedo G, Roberts CW, Carrero JC, Morales-Montor J. Parasite regulation by host hormones: an old mechanism of host exploitation? See comment in PubMed Commons below Trends Parasitol. 2005; 21: 588-593.
- Morales-Montor J, Hall CA. The host-parasite neuroimmunoendocrine network in schistosomiasis: consequences to the host and the parasite. See comment in PubMed Commons below Parasite Immunol. 2007; 29: 599-608.
- Hernández-Bello R, Escobedo G, Guzmán C, Ibarra-Coronado EG, López-Griego L, Morales-Montor J, et al. Immunoendocrine host-parasite interactions during helminth infections: from the basic knowledge to its possible therapeutic applications. See comment in PubMed Commons below Parasite Immunol. 2010; 32: 633-643.
- Zaccone P, Burton OT, Cooke A. Interplay of parasite-driven immune responses and autoimmunity. See comment in PubMed Commons below Trends Parasitol. 2008; 24: 35-42.
- van der Kleij D, Latz E, Brouwers JF, Kruize YC, Schmitz M, Kurt-Jones EA, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. See comment in PubMed Commons below J Biol Chem. 2002; 277: 48122-48129.
- Nono JK, Pletinckx K, Lutz MB, Brehm K. Excretory/secretory-products of Echinococcus multilocularis larvae induce apoptosis and tolerogenic properties in dendritic cells in vitro. See comment in PubMed Commons below PLoS Negl Trop Dis. 2012; 6: e1516.
- Adalid-Peralta L, Fleury A, García-Ibarra TM, Hernández M, Parkhouse M, Crispín JC, et al. Human neurocysticercosis: in vivo expansion of peripheral regulatory T cells and their recruitment in the central nervous system. See comment in PubMed Commons below J Parasitol. 2012; 98: 142-148.
- Adalid-Peralta L, Arce-Sillas A, Fragoso G, Cárdenas G, Rosetti M, Casanova-Hernández D, et al. Cysticerci drive dendritic cells to promote in vitro and in vivo Tregs differentiation. See comment in PubMed Commons below Clin Dev Immunol. 2013; 2013: 981468.
- Nava-Castro K, Hernández-Bello R, Muñiz-Hernández S, Camacho-Arroyo I, Morales-Montor J. Sex steroids, immune system, and parasitic infections: facts and hypotheses. See comment in PubMed Commons below Ann N Y Acad Sci. 2012; 1262: 16-26.