
Review Article
Austin J Clin Neurol. 2024; 11(1): 1165.
Nerve Regeneration After Peripheral Nerve Injury, Are Flavonoids the Answer? A Scoping Review
Nieto L1*; Hernández AM2; López-Sarasty L2; Camacho-Domínguez L3
1Department of Plastic Surgery, Fundación Santa Fe de Bogotá, Colombia
2Pontifical Javeriana University, Colombia
3Department of Plastic Surgery, Pontifical Javeriana University, Colombia
*Corresponding author: Nieto L Department of Plastic Surgery, Fundación Santa Fe de Bogotá. Cl. 123 #7A -60, Bogotá, Colombia. Tel: +57310 7631576 Email: luis.nieto@fsfb.org.co
Received: March 06, 2024 Accepted: April 18, 2024 Published: April 25, 2024
Abstract
Flavonoids are polyphenol compounds of plants origin with antioxidant, anti-inflammatory, immunomodulatory, and analgesic functions, among others. Peripheral nerve lesions represent a burden for patients and a high cost for the health systems. Although a diversity of surgical management is available, adjuvant therapies are required to improve regeneration time and prevent atrophy of the affected target organs. This review aims to synthesize the available information on the use of flavonoids in peripheral nerve regeneration. A review of the available literature was carried out, demonstrating that flavonoids are a therapeutic alternative that could favor axonal regeneration. However, clinical studies are required since the available works reviewed are experimental in murine models, and their extrapolation to humans is essential.
Keywords: Flavonoids; Peripheral nerve injury; Nerve regeneration; Schwann cell
Abbreviations: PNL: Peripheral Nerve Lesions; 7,8 DHF: 7,8-Dihydroxyflavone; BDNF: Neurotrophin Brain-Derived Neurotrophic Factor; trkB: Tropomyosin Receptor Kinase B; GDNF: Glia-Derived Neurotrophic Factor; BYHWD: Buyang Huanwu; GAP-43: Proteins Growth-Associated Protein 43; EGCG: Epigallocatechin-3-Gallate; NGF: Neural Growth Factor; SC: Schwann cells; Nrf2: Nuclear Factor Erythroid 2-Factor 2
Introduction
Peripheral Nerve Lesions (PNL) are a group of entities that include sensory and motor nerve disorders. It has been estimated that 20 million people are affected in the US, generating an average cost of $150,000 annually. This type of injury is associated with high disability depending on the location and type of injury, leading even to loss of the limb function. Among the most frequent mechanisms of injury are trauma, compression, iatrogenic lesions, metabolic and infectious alterations. It should be noted that trauma is the most frequent, with a prevalence of 1.3-2.8% [1,2].
There are different therapeutic options for its treatment, in which microsurgical reconstruction stands out. However, it must be considered that up to a third of the patients who receive the surgical intervention will have alterations in their functionality and quality of life related to incomplete recovery. Taking this into account, the need for new treatments that promote cell regeneration is imperative [3]. Among the available options, mesenchymal cells and bone marrow transplantation aim to promote cell differentiation processes, metabolic activity, and growth factors for post-injury repair [4,5]. Recently, flavonoids have been described as a novel tool to promote regeneration [6-10].
Flavonoids are secondary metabolites of plant-based polyphenols, which promote pigmentation and herbal reproduction and are found primarily in fruits and vegetables [11,12]. Multiple benefits have been attributed to them since they have anti-inflammatory, immunomodulatory, and antioxidant effects, among others [12]. They are used in neurodegenerative diseases and as an adjuvant in central nervous system lesions. When discussing its use in nerve regeneration after a peripheral nerve injury, there is literature available describing its positive association, thus being a possible treatment option [6-10]. Considering the above, this review aims to synthesize the available information about the use of flavonoids in peripheral nerve regeneration.
Methods
A search of the literature was performed in the electronic databases PUBMED, EMBASE, COCHRANE, and LILACS with the terms "flavonoidal" OR "flavonoid" OR "flavonoids" OR "flavonoidic" OR "flavonoids" [MeSH Terms] OR "flavonoid" AND ("peripheral nerve injuries"[MeSH Terms] OR ("nerve regeneration"[MeSH Terms]. No discrimination was made based on study design or publication date. The articles found were extracted to Rayyan software for later review, and duplicates were eliminated.
Eligibility Criteria
Articles included in this review had to use any molecule belonging to the flavonoid family as an intervention to assess its effect on peripheral nerve regeneration. When evaluating nerve regeneration, articles could use any measurement method, including different motor, sensory, and morphological evaluation tools. Articles had to be in Spanish or English. Articles that did not describe the methods used or for which the complete manuscript could not be obtained were excluded.
Article Selection Process
Three reviewers independently evaluated titles and abstracts of the manuscripts based on the eligibility criteria. Once discriminated, selection differences were resolved in a group reunion. Only those articles that the three reviewers approved were included. Subsequently, the complete manuscripts were analyzed, and data extraction was made. As a guide during the identification, review, and selection process, the PRISMA - ScR guidelines were followed [13].
Data Extraction
Three reviewers independently assessed the full articles and extracted the title, authors, year of publication, study design, country, and research objectives. Additionally, the type of flavonoid used in the intervention, the study subjects, the methodology and tools for measuring nerve regeneration, results, conclusions, and limitations of each study were recorded. All this information was recorded in Excel © 2022 Microsoft.
Results
Selection of Sources of Evidence
After searching for articles in the electronic databases, 49 articles were obtained. Six duplicate articles were found. Subsequently, the review of titles and abstracts was carried out by applying the eligibility criteria, where 25 papers were selected that met all the characteristics. After reviewing the complete articles, those that did not have the entire manuscript or did not report the method for evaluating regeneration were excluded. Finally, 23 articles remained for analysis (Figure 1) [10,14-35].
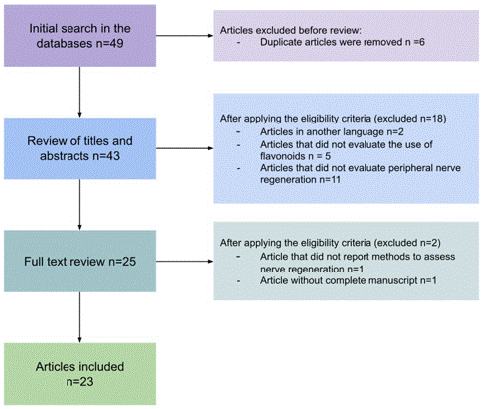
Figure 1: Article selection process.
Characteristics of Sources of Evidence
Intervention
In the selected medical literature, 12 different types of flavonoids were found to favor peripheral nerve regeneration, 7,8-dihydroxyflavone (7,8 DHF), baicalin, bogijetong, buyang huanwu, epigallocatechin-3-gallate, epimedium, myricetin, naringin, pueraria lobata, quercetin, red propolis, and silymarin.
7,8-Dihydroxyflavone (7,8 DHF)
One of the essential growth-promoting molecules used for axonal regeneration in peripheral nerves is the neurotrophin Brain-Derived Neurotrophic Factor (BDNF), which promotes Schwann Cell (SC) transformation and stimulates neuritic elongation by binding to receptors from the tropomyosin receptor kinase B (trkB). In vitro experiments show that this molecule binds to the trkB receptor with high affinity, producing tyrosine phosphorylation and activating the same cascade of BDNF signaling components. The magnitude of these molecular changes is like those observed in rodents with sciatic nerve lesions treated with recombinant human BDNF, neurotrophin 4/5, electrical stimulation, or treadmill exercise, which share the mechanism by which they increase trkB signaling in regenerated axons. It has been shown that injections or topical application of 7,8 DHF at the time of nerve repair promote axonal regeneration, increasing the elongation of regenerated axons and improving muscle reinnervation [34].
Baicalin
Scutellaria baicalensis Georgii is a plant used to treat inflammatory processes, fever, ulcers, cancer, prophylaxis and treatment of cardiovascular, gastrointestinal, and immune diseases [36]. Clinical observations have shown its effectiveness in treating nervous system excitability conditions, neurosis, and insomnia [37]. Results of recent studies report that the flavonoids present in its leaves and roots have neuroprotective effects, specifically Baicalin, with a wide variety of biological functions, with anti-inflammatory, antioxidant, and anti-apoptotic effects (38). Previous studies have reported its neuroprotective effect in rats, with the ability to promote neuronal differentiation of neural stem cells [39]. It has been demonstrated that significantly improves SC survival and function in vitro via upregulation of the S100 protein, a potentially critical factor contributing to neuronal development and stimulating the expression of Glia-Derived Neurotrophic Factor (GDNF), BDNF and Ciliary Neurotrophic Factor [15]. In other studies, the addition of Baicalin to culture media modulates the growth of cells derived from the dorsal root ganglion in the number and length of their neurites, accompanied by migration of SC, which suggests better survival of the cultured neurons and greater branching of the regenerated neurites, probably dependent on the presence of antioxidant flavonoids [35].
Bogijetong-Tang
Bogijetong-tang has been used in treating neuropathic pain and regulating serum glucose levels in diabetic patients in traditional Asian medicine [40]. It has been reported as a promoter of regenerative responses in spinal cord injuries, as well as in axonal regeneration after PNL and neuronal survival associated with Schwann cell activation [41-43]. Other studies show a protective effect in neuropathic lesions such as taxol injection or crush lesions in peripheral nerves [16].
Buyang Huanwu (BYHWD)
Overexpression of the Hsp27 protein in rodent studies have shown acceleration of axonal regeneration and motor recovery after prolonged injury, implying a potential application in human patients [44]. On the other hand, it has been reported that BYHWD protects SC from oxidative stress, mediates regenerative responses after spinal cord injury, and axonal regeneration in sciatic nerve injuries, acting on regenerated axons and SC [45,46]. It has been shown that treatment with BYHWD over-regulates proteins Growth-Associated Protein 43 (GAP-43) production in injured axons and activates Cdc2 signaling in SC. Sensory and motor activity results were significantly high in rats treated for prolonged periods. In humans, these results are lower, probably because the distances that regenerated axons must travel are much longer, and human SC at the distal ends of the lesions are less responsive, especially in prolonged denervation [22].
Epigallocatechin-3-Gallate (EGCG)
EGCG is the most abundant polyphenol in green tea, it has shown to have neurological effects, improve cognitive function and learning abilities, and prevent cardiovascular diseases and cancer. Other effects have shown that it slows the progression of neurodegenerative disorders due to a potential to inhibit neuronal apoptosis triggered by neurotoxic activities such as oxidative stress and neuroinflammation. In addition, it protects muscle fibers from cell death by activating anti-apoptotic signaling and inhibiting overexpression of the apoptotic factors as p53. It improves morphological recovery in skeletal muscle tissues after peripheral nerve injuries in rats, demonstrated by histological sections where reduced thickness and aggregate of connective tissue in the muscle fibers of treated animals is observed and significant progression of the response [17]. The administration of green tea extract to rats improves motor and sensitive reflexes and reduces hyperalgesia and allodynia in chronic compressive lesions of the sciatic nerve [47]. There is evidence that EGCG can reduce NADPH-d/nNOS reactivity and improve the survival of motor neurons in the injured hypoglossal nucleus and vagus nerve [48]. There is clear evidence that EGCG administration accelerates the recovery of motor and sensory function in sciatic nerve injuries, improving their regeneration in vivo, it significantly reduces the ultrastructural histological changes associated with nerve injury, indicating rapid cell regeneration with decreased signs of apoptosis, these characteristics suggest a neuroprotective mechanism against functional losses and neuropathological alterations seen in PNL [19]. The therapeutic effects are associated to the up regulation of various neurotrophic factors such as BDNF, GDNF, and NT3 [26]. Recent studies have demonstrated another effect of EGCG by finding an up regulation of glutathione reductase that supports the idea that it acts in an indirect pathway for the induction of enzymes and transcription factors of neuronal regeneration. In experiments with regenerative tubes to treat segmental losses of peripheral nerves, this compound has been added showing its antioxidant and anti-inflammatory effects during the whole recovery period [10] Also, it improves the regeneration of the treated peripheral nerves and a good regenerative process of the gastrocnemius muscle affected in sciatic nerve lesions [20]. Less adhesion of nerves to surrounding tissues has been found, probably due to its anti-inflammatory effects. It also promotes the regeneration of blood vessels at the injury and around the nerve repair. Improvement of the muscle weight affected by nerve injury has been shown due to faster regeneration compared to the control study groups and increased nerve conduction velocity in electromyography studies, indicating that it prevents muscle atrophy. The expression of the GAP-43 protein has been detected in animals. A more significant number of myelinated fibers of the repaired nerves was found, greater thickness of the myelin layer, and normal morphology of the SCs has been found [23].
Epimedium
Epimedium extract has been used in China to treat erectile dysfunction, postmenopausal syndrome, and osteoporosis for hundreds of years, Icariin being its main component. It has been shown to promote bone formation and have neurotrophic effects in in vivo and in vitro experiments [49,50]. It has also been found to promote muscle function recovery after spinal cord injury in rats [51]. Other studies suggest that since nerve regeneration is a sum of factors, these compounds promote its development, although the details of their mechanisms of action are still unclear [21]. A more recent study found that local application of Icariin contributes to peripheral nerve regeneration, increasing the number of regenerated nerve fibers and nerve conduction velocity. Also, improving functional recovery measured and comparative histopathological changes [27].
Myricetin
It is a plant-based flavonoid (3,3',4',4,5,5,5',7-hexahydroxyflavon) present in various vegetables, fruits, nuts, and red wine. It has been reported to have antioxidant, antibacterial, cardioprotective, and antiproliferative properties [52]. A recent study investigated its effects after sciatic nerve injury in rats, showing significantly high values in the sciatic functional index and the arthroscopic position index and recovery of sensorimotor functions. The expression of BDNF and TrkB factors was also established, reflected in a more remarkable axonal regeneration and myelination [29].
Naringin (4’,5,7-Trihidroxi Flavonone 7-Ramnoglucosida)
Naringin is a flavonoid glycoside in grapefruit, tomato, and citrus fruits. It has been shown to significantly increase SC proliferation after neurectomy of sciatic nerve [33]. Also, acute and chronic pain disorders such as hyperalgesia after PNL have been significantly reduced by treatment with naringin due to its antioxidant properties which act as a non-enzymatic cellular defense, maintaining cellular homeostasis by reducing oxygen radicals and by its inhibitory effect of neurotrophin p75, promoting axonal regeneration and functional recovery [53-55]. Other studies have found that it suppresses the activation of c-Jun N-terminal protein kinase, preventing cell damage, which is demonstrated in the improvement of sciatic function index tests [30].
Pueraria Lobata
Puerarin has been used in traditional Chinese medicine for treatment of various diseases, including nerve diseases, for its effects as a free radical scavenger, antioxidant, inhibition of inflammatory response and reperfusion, and improvement of cerebral microcirculation, suggesting that it may reduce the initial damage in PNL and facilitate their repair. GAP43 is a membrane-associated phosphorylated protein related to neuronal growth, neurite formation, and brain plasticity, in the study groups treated with puerarin, GAP43 overexpression was found in the injured segments, where it was observed that the number, integrity, and thickness of the myelin sheaths of the regenerated axons were more significant, confirmed by histological findings, and the muscle mass index [31]. Its oral administration has been shown to promote regeneration of injured sciatic nerves and promoting axonal growth, metabolites of Pueraria lobata have shown nerve differentiation effects in vitro, increasing the number of neurite-bearing cells and expression of synapsin I in cell cultures supplemented with NGF. Quality of nerve regeneration and its morphology compared between study groups confirm that nerve structures are more mature with higher values of total nerve area, endoneuria area, and number of myelinated axons. Daidzein, principal metabolite, has been found to bind to estrogen receptors and exhibits estrogenic activity, markedly accelerating axonal growth, additionally, daidzein has an immune stimulatory effect, increasing phagocytic response of macrophages and altering prostaglandin synthesis, producing degeneration of the damage nerve, and releasing neurotrophic factors that accelerate the maturation of regenerated nerves [18].
Quercetin
Quercetin shows beneficial effects in several in vivo models of neural disorders, such as brain trauma, spinal cord injury, and cerebral ischemia [56]. In vitro studies also reveal increase neuronal survival and decrease of toxicity and neuroinflammation [57]. Also induces neurite outgrowth by promoting GAP-43 expression, increased cAMP expression, and remyelination after sciatic nerve injury in rats [25,58] Intracellular cAMP levels are directly associated with activation of intrinsic axonal growth capacity, the overregulation of IL-6, which, through STAT3, induces regeneration-related genes, such as GAP43 [32]. Treatment with quercetin shows significant recovery after PNL, demonstrated by increased motor functional indexes, reflecting axonal integrity and number of reinnervated fibers in target muscles and the elongation of regenerated neurites stimulated by synaptotagmin-1, as well as increased diameter and thickness of the myelin sheath. More recent studies show that it promotes the expression of myelin-associated glycoprotein and peripheral myelin protein 22, which benefit remyelination, in addition to promoting SC proliferation and migration, which reflects the suppression of oxidative stress by reducing the production of Nox4 and Duox1 and promoting the expression of Nuclear Factor Erythroid 2-Factor 2 (Nrf2) and SOD2, both proteins involved with inhibition of free radical production [25,59]. It reduces oxidative stress and inflammatory response, attenuating neuronal autophagy and apoptosis [28].
Red Propolis
Red propolis plant extract contains antioxidant agents, including phenols, flavonoids, naphthoquinones, and others. It has shown several biological activities, including cytotoxic activity against cell tumors and antimicrobial properties. These antioxidant agents help to clear myelin by macrophages and thus accelerate the regenerative process of the injured nerve [60]. Other studies evidence their neuroprotective effects, improving sensory and motor recovery, decreasing inflammation, and increasing myelin axons [24].
Silymarin
Silibum marianum fruit extract comprises one flavonoid, taxifolin, and seven flavones. Antioxidant effects are attributed to it, such as direct scavenging of free radicals and chelation of free Fe and Cu, preventing their formation and inhibiting specific enzymes producing them and protecting the integrity of mitochondria under stress conditions, maintaining an optimal intracellular balance by activation of a variety of antioxidant enzymes and non-enzymatic antioxidants mainly through Nrf2 pathway [61]. These characteristics can be demonstrated with the improvement of axonal regeneration and CS, accompanied by the myelination process and structural recovery of the regenerated nerve fibers [14].
Discussion
Flavonoids have existed in nature for about a billion years, interacting with evolving organisms. They are defined as a broad compound of polyphenols, origin in the plant kingdom and found in vascular plants due to their secondary metabolism. They are low molecular weight compounds that carry numerous functions, among as protective agents against UV light, anti-inflammatory, analgesic, anti-allergic, venotonic action, and especially their potent antioxidant activity. Its function has recently been studied in other fields, such as mental health and the nervous system, with positive effects on nerve signaling and the function of molecular receptors in the central nervous system [62-64].
As a matter of fact, injured nerves in the peripheral nervous system can regenerate and reinnervate their target organs [65]. After an injury, approximately one-third of patients remain with incomplete functional recovery. Furthermore, it is associated with chronic pain, muscle atrophy, and weakness, resulting in lifelong disability and costs in medical follow-up [14]. Advanced microsurgical techniques have established effective treatment for this entity, but adjuvant therapies are required to improve regeneration times and prevent atrophy of the target organs. Recently, flavonoids have been extensively studied, finding beneficial effects to complement or improve different treatments, given their antioxidant and anti-inflammatory capacity [66].
The present study evaluated the available literature to determine if flavonoids after PNL could be a treatment that benefits nerve regeneration and improves outcomes. Many studies, both in vitro and in vivo, have shown the positive effect of flavonoids in the regeneration of the peripheral nervous system, evaluating sensorimotor recovery. These effects are related to cell signaling pathways that reduce neuroinflammation and oxidative stress and promote the expression of neurotrophic and axonal protection factors such as BDNF, NGF and TrkB. The molecular mechanisms are extensive and still not fully clarified; however, most studies have shown that flavonoids, due to their neuroprotective, anti-inflammatory, and anti-apoptotic effect, decrease the degree of inflammation and increase the number, integrity, and thickness of the pods of myelin, which improves functional recovery compared to placebo [24]. Recovery of normal neurological function remains a significant challenge, however, flavonoids could be an effective adjuvant therapy to improve axonal regeneration and sensorimotor recovery.
The diversity of evaluated studies and origin countries demonstrates the growing interest in flavonoids to improve axonal regeneration in PNL. China has the most studies, probably due to the massive consumption of flavonoids for different purposes. Most articles were experimental studies in a murine model, showing the ease of this type of study in these animals. Nevertheless, similar studies in large animals are necessary to explore the optimal treatment conditions, maximize the translational potential of these molecules, and extrapolate results to humans.
Limitations
To date, this article is the first review of the use of flavonoids in nerve regeneration available in the literature. Articles published in different languages were included, and multiple databases were searched to reduce publication bias. A possible limitation of this work is that no review of the gray literature was carried out.
Conclusions
PNL is associated with high costs to health system and high disability. Advances in nerve repair require adjuvant therapies to reduce care costs and improve sensory, motor, and proprioceptive recovery after the injury. Based on what has been observed, flavonoids are a therapeutic alternative that could favor nerve regeneration, given their anti-inflammatory and antioxidant function. Since the articles evaluated are experimental studies, additional controlled clinical studies are required to determine the effectiveness of the different types of flavonoids in nerve regeneration, their mechanisms of action, the factors involved, and their safety.
Author Statements
Financing
This article does not have external sponsorship. It was financed with the researcher’s funds.
Conflicts of Interest
None of the researchers declared any conflict of interest with the topic to be discussed.
References
- Grinsell D, Keating CP. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. BioMed Research International. 2014; 2014: 1-13.
- Sullivan R, Dailey T, Duncan K, Abel N, Borlongan C. Peripheral nerve injury: Stem cell therapy and peripheral nerve transfer. International Journal of Molecular Sciences. 2016; 17: 2101.
- Lundborg G. Nerve injury and repair - a challenge to the plastic brain. Journal of the Peripheral Nervous System 2003; 8: 209-226.
- Lavorato A, Raimondo S, Boido M, Muratori L, Durante G, Cofano F, et al. Mesenchymal stem cell treatment perspectives in peripheral nerve regeneration: Systematic review. International Journal of Molecular Sciences. 2021; 22: 572.
- Li W, Jia H, Wang ZD, Zhai F, Sun G da, Ma D, et al. Combinatory transplantation of mesenchymal stem cells with flavonoid small molecule in acellular nerve graft promotes sciatic nerve regeneration. J Tissue Eng. 2020: 11: 2041731420980136.
- Haque AM, Hashimoto M, Katakura M, Hara Y, Shido O. Green tea catechins prevent cognitive deficits caused by Aβ1-40 in rats. Journal of Nutritional Biochemistry. 2008; 19: 619–26.
- Shirai N, Suzuki H. Effect of Dietary Docosahexaenoic Acid and Catechins on Maze Behavior in Mice. Ann Nutr Metab. 2004; 48: 51–8.
- Unno K, Takabayashi F, Kishido T, Oku N. Suppressive effect of green tea catechins on morphologic and functional regression of the brain in aged mice with accelerated senescence (SAMP10). Exp Gerontol. 2004; 39: 1027–34.
- van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, et al. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. Journal of Neuroscience. 2007; 27: 5869–78.
- Renno WM, Benov L, Khan KM. Possible role of antioxidative capacity of (-)-epigallocatechin-3-gallate treatment in morphological and neurobehavioral recovery after sciatic nerve crush injury. J Neurosurg Spine. 2017; 27: 593–613.
- Serafini M, Peluso I, Raguzzini A. Flavonoids as anti-inflammatory agents. In: Proceedings of the Nutrition Society. 2010; 273–8.
- Wen L, Jiang Y, Yang J, Zhao Y, Tian M, Yang B. Structure, bioactivity, and synthesis of methylated flavonoids. Annals of the New York Academy of Sciences. Blackwell Publishing Inc. 2017; 1398: 120–9.
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Vol. 169, Annals of Internal Medicine. American College of Physicians. 2018; 467–73.
- Ebrahimi-Zadehlou P, Najafpour A, Mohammadi R. Assessments of regenerative potential of silymarin nanoparticles loaded into chitosan conduit on peripheral nerve regeneration: a transected sciatic nerve model in rat. Neurol Res. 2021; 43: 148–56.
- Zuo W, Wu H, Zhang K, Lv P, Xu F, Jiang W, et al. Baicalin promotes the viability of Schwann cells in vitro by regulating neurotrophic factors. Exp Ther Med. 2017; 14: 507–14.
- Ahn SH, Chang IA, Kim KJ, Kim CJ, Namgung U, Cho CS. Bogijetong decoction and its active herbal components protect the peripheral nerve from damage caused by taxol or nerve crush. BMC Complement Altern Med. 2016; 16.
- Renno WM, Al-Maghrebi M, Al-Banaw A. (-)-Epigallocatechin-3-gallate (EGCG) attenuates functional deficits and morphological alterations by diminishing apoptotic gene overexpression in skeletal muscles after sciatic nerve crush injury. Naunyn Schmiedebergs Arch Pharmacol. 2012; 385: 807–22.
- Chen HT, Yao CH, Chao PDL, Hou YC, Chiang HM, Hsieh CC, et al. Effect of serum metabolites of Pueraria lobata in rats on peripheral nerve regeneration: In vitro and in vivo studies. J Biomed Mater Res B Appl Biomater. 2008; 84: 256–62.
- Renno WM, Al-Maghrebi M, Alshammari A, George P. (-)-Epigallocatechin-3-gallate (EGCG) attenuates peripheral nerve degeneration in rat sciatic nerve crush injury. Neurochem Int. 2013; 62: 221–31.
- Qian Y, Yao Z, Wang X, Cheng Y, Fang Z, Yuan WE, et al. (-)-Epigallocatechin gallate-loaded polycaprolactone scaffolds fabricated using a 3D integrated moulding method alleviate immune stress and induce neurogenesis. Cell Prolif. 2020; 53.
- Kou Y, Wang Z, Wu Z, Zhang P, Zhang Y, Yin X, et al. Epimedium extract promotes peripheral nerve regeneration in rats. Evidence-based Complementary and Alternative Medicine. 2013; 2013: 1-6.
- Kim KJ, Namgung U. Facilitating effects of Buyang Huanwu decoction on axonal regeneration after peripheral nerve transection. J Ethnopharmacol. 2018; 213: 56–64.
- Chen J, Yang R, Li H, Lao J. Green tea polyphenols promote functional recovery from peripheral nerve injury in rats. Medical Science Monitor. 2020; 26: 1-9.
- Barbosa RA, Nunes TLGM, Nunes TLGM, Paixão AO da, Neto RB, Moura S, et al. Hydroalcoholic extract of red propolis promotes functional recovery and axon repair after sciatic nerve injury in rats. Pharm Biol. 2016; 54: 993–1004.
- Qiu J, Yang X, Wang L, Zhang Q, Ma W, Huang Z, et al. Isoquercitrin promotes peripheral nerve regeneration through inhibiting oxidative stress following sciatic crush injury in mice. Ann Transl Med. 2019; 7: 680–680.
- Renno WM, Khan KM, Benov L. Is there a role for neurotrophic factors and their receptors in augmenting the neuroprotective effect of (-)-epigallocatechin-3-gallate treatment of sciatic nerve crush injury? Neuropharmacology. 2016; 102: 1–20.
- Chen B, Niu SP, Wang ZY, Wang ZW, Deng JX, Zhang PX, et al. Local administration of icariin contributes to peripheral nerve regeneration and functional recovery. Neural Regen Res. 2015; 10: 84–9.
- Huang C, Fu C, Qi ZP, Guo WL, You D, Li R, et al. Localised delivery of quercetin by thermo-sensitive PLGA-PEG-PLGA hydrogels for the treatment of brachial plexus avulsion. Artif Cells Nanomed Biotechnol. 2020; 48: 1010–21.
- Zhang D, Ge J, Ye L, Tang H, Bai X. Myricetin promotes peripheral nerve regeneration in rat model of sciatic nerve injury via regulation of BDNF-AKt/GSK-3β/mTOR signalling pathway. Tropical Journal of Pharmaceutical Research. 2018; 17: 2355–63.
- Oliveira MA, Heimfarth L, Passos FRS, Miguel-dos-Santos R, Mingori MR, Moreira JCF, et al. Naringenin complexed with hydroxypropyl-β-cyclodextrin improves the sciatic nerve regeneration through inhibition of p75NTR and JNK pathway. Life Sci. 2020; 241.
- Wu MF, Zhao GJ, Yang XY, Peng CG, Zhao JW, Liu J, et al. Puerarin accelerates neural regeneration after sciatic nerve injury. Neural Regen Res. 2014; 9: 589–93.
- Chen MM, Qin J, Chen SJ, Yao LM, Zhang LY, Yin ZQ, et al. Quercetin promotes motor and sensory function recovery following sciatic nerve-crush injury in C57BL/6J mice. Journal of Nutritional Biochemistry. 2017; 46: 57–67.
- Samadian H, Vaez A, Ehterami A, Salehi M, Farzamfar S, Sahrapeyma H, et al. Sciatic nerve regeneration by using collagen type I hydrogel containing naringin. J Mater Sci Mater Med. 2019; 30.
- English AW, Liu K, Nicolini JM, Mulligan AM, Ye K. Small-molecule trkb agonists promote axon regeneration in cut peripheral nerves. Proceedings of the National Academy of Sciences. 2013; 110: 16217–22.
- Shurygin AYa, Bezzubov NV, Ignatova EA, Nikolaev SM, Viktorov IV. Bulletin of Experimental Biology and Medicine. 2002; 134: 47–9.
- Pshenichkina YuA. Biology of scutellaria baicalensis Georgi (Lamiaceae) from different ecological and geographical places of growth during introduction. Contemporary Problems of Ecology. 2022; 15: 653–8.
- Shurygin Aya, Bezzubov NV, Ignotava EA, Nikolaev SM, Viktrov IV. Bulletin of experimental Biology and Medicine. 2002; 134: 47-9.
- Miao G, Zhao H, Guo K, Cheng J, Zhang S, Zhang X, et al. Mechanisms underlying attenuation of apoptosis of cortical neurons in the hypoxic brain by flavonoids from the stems and leaves of scutellaria baicalensis Georgi. Neural Regen Res. 2014; 9: 1592–8.
- Tu XK, Yang WZ, Shi SS, Wang CH, Chen CM. Neuroprotective effect of baicalin in a rat model of permanent focal cerebral ischemia. Neurochem Res. 2009; 34: 1626–34.
- Kim JM, Cho CS. Clinical study of 8 diabetic patients with paresthesia. The Journal of Internal Korean Medicine. 2010; 31: 372–9.
- Ma J, Li W, Tian R, Lei W. Ginsenoside Rg1 promotes peripheral nerve regeneration in rat model of nerve crush injury. Neurosci Lett. 2010; 478: 66–71.
- Tae BS, Baek K, Kwon KB, Lee SI, Lim JS, In CS, et al. Shengmai-san - Mediated enhancement of regenerative responses of spinal cord axons after injury in rats. J Pharmacol Sci. 2009; 110: 483–92.
- Wang L, Yuan D, Zhang D, Zhang W, Liu C, Cheng H, et al. Ginsenoside Re Promotes Nerve Regeneration by Facilitating the Proliferation, Differentiation and Migration of Schwann Cells via the ERK- and JNK-Dependent Pathway in Rat Model of Sciatic Nerve Crush Injury. Cell Mol Neurobiol. 2015; 35: 827–40.
- Ma CHE, Omura T, Cobos EJ, Latrémolière A, Ghasemlou N, Brenner GJ, et al. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. Journal of Clinical Investigation. 2011; 121: 4332–47.
- Chang IA, Lim HD, Kim KJ, Shin H, Namgung U. Enhanced axonal regeneration of the injured sciatic nerve by administration of Buyang Huanwu decoction. J Ethnopharmacol. 2016; 194: 626–34.
- Chen A, Wang H, Zhang J, Wu X, Liao J, Li H, et al. BYHWD rescues axotomized neurons and promotes functional recovery after spinal cord injury in rats. J Ethnopharmacol. 2008; 117: 451–6.
- Renno WM, Saleh F, Klepacek I, Al-Khaledi G, Ismael H, Asfar S. Green tea pain modulating effect in sciatic nerve chronic constriction injury rat model. Nutr Neurosci. 2006; 9: 41–7.
- Wei IH, Tu HC, Huang CC, Tsai MH, Tseng CY, Shieh JY. (-)-Epigallocatechin gallate attenuates NADPH-d/nNOS expression in motor neurons of rats following peripheral nerve injury. BMC Neurosci. 2011; 12.
- Ma H, He X, Yang Y, Li M, Hao D, Jia Z. The genus Epimedium: An ethnopharmacological and phytochemical review. Vol. 134, Journal of Ethnopharmacology. 2011; 519–41.
- Shindel AW, Xin ZC, Lin G, Fandel TM, Huang YC, Banie L, et al. Erectogenic and neurotrophic effects of icariin, a purified extract of horny goat weed (Epimedium spp.) in vitro and in vivo. Journal of Sexual Medicine. 2010; 7: 1518–28.
- Tohda C, Nagata A. Epimedium koreanum extract and its constituent icariin improve motor dysfunction in spinal cord injury. Evidence-based Complementary and Alternative Medicine. 2012; 2012: 1-6.
- Zhang XH, Chen SY, Tang L, Shen YZ, Luo L, Xu CW, et al. Myricetin Induces Apoptosis in HepG2 Cells Through Akt/p70S6K/Bad Signaling and Mitochondrial Apoptotic Pathway. Anticancer Agents Med Chem. 2013; 13: 1575-81.
- Kaulaskar S, Bhutada P, Rahigude A, Jain D, Harle U. Effects of naringenin on allodynia and hyperalgesia in rats with chronic constriction injury-induced neuropathic pain. Journal of Chinese Integrative Medicine. 2012; 10: 1482-9.
- Zhang Y, Liu B, Chen X, Zhang N, Li G, Zhang LH, et al. Naringenin Ameliorates Behavioral Dysfunction and Neurological Deficits in a d-Galactose-Induced Aging Mouse Model Through Activation of PI3K/Akt/Nrf2 Pathway. Rejuvenation research. 2017; 20: 462-72.
- Wang YT, Lu XM, Zhu F, Huang P, Yu Y, Long ZY, et al. Ameliorative Effects of p75NTR-ED-Fc on Axonal Regeneration and Functional Recovery in Spinal Cord-Injured Rats. Mol Neurobiol. 2015; 52: 1821–34.
- Schültke E, Kendall E, Kamencic H, Ghong Z, Griebel Rw, Juurlink BH. Quercetin promotes functional recovery following acute spinal cord injury. J Neurotrauma. 2004; 20: 583–91.
- Kraus B, Wolff H, Heilmann J, Elstner EF. Influence of Hypericum perforatum extract and its single compounds on amyloid-β mediated toxicity in microglial cells. Life Sci. 2007; 81: 884–94.
- Chen M, Yin ZQ, Zhang LY, Liao H. Quercetin promotes neurite growth through enhancing intracellular camp level and gap-43 expression. Chinese Journal of Natural Medicines. 2015; 13: 667-72.
- Palazzolo G, Zenobi-Wong M, Horvath P. The Flavonoid Isoquercitrin Promotes Neurite Elongation by Reducing RhoA Activity. PLoS One. 2012; 7: 1-10.
- Da Silva Frozza CO, Garcia CSC, Gambato G, de Souza MDO, Salvador M, Moura S, et al. Chemical characterization, antioxidant and cytotoxic activities of Brazilian red propolis. Food and Chemical Toxicology. 2013; 52: 137–42.
- Surai P. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants. 2015; 4: 204–47.
- Cartaya O, Reynaldo I. Flavonoides: Características químicas y aplicaciones. Cultivos tropicales. 2001: 2: 5-14.
- López luengo MT. Flavonoides. OFF ARM. 2002; 21: 108–13.
- Estrada-Reyes R, Ubaldo-Suárez D, Araujo-Escalona AG. Los flavonoides y el Sistema Nervioso Central. Salud Mental 2012; 35: 375-384.
- Gordon T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int J Mol Sci. 2020; 21: 8652.
- Muratori L, Fregnan F, Maurina M, Haastert-Talini K, Ronchi G. The Potential Benefits of Dietary Polyphenols for Peripheral Nerve Regeneration. Int J Mol Sci. 2022; 23: 1-31.