
Research Article
Austin J Clin Neurol. 2024; 11(1): 1166.
Eye Movements Features in Individuals with Amnestic Mild Cognitive Impairment: Preliminary Findings Based on a Head-mounted Intelligent Analysis System
Yun Huang1-3; Shuyun Huang3; Xia Xiang3; Pan Shang3; Chunyan Zhang3; Yajing Liu3; Meiqiu Li3; Jiali Luo3; Xiaoying Zhong3; Haiqun Xie3*; Hongzhen Zhou1*;
1Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, China
2School of Nursing, Southern Medical University, Guangzhou, Guangdong, China
3First People’s Hospital of Foshan, Foshan, Guangdong, China
*Corresponding author: Haiqun Xie, M.Med First People’s Hospital of Foshan, Foshan, Guangdong, China; Hongzhen Zhou, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, China. Email: haiqunx@foxmail.com; 913860896@QQ.com
Received: March 19, 2024 Accepted: April 24, 2024 Published: May 01, 2024
Abstract
Objective: This study aimed first to investigate the eye movement features of patients with amnestic mild cognitive impairment (aMCI) tested by a head-mounted intelligent analysis system and then explore the association between abnormal eye movement and aMCI.
Method: In this cross-sectional study, sixty-six participants were included, and forty (21 cognitively normal, CN; 19 aMCI) were analyzed finally. Neuropsychological battery tests were conducted to assess cognitive function, including global cognition and cognitive domains. Eye movement parameters were recorded using a head-mounted intelligent analysis system. The eye movement tasks contained fixation, smooth pursuit (horizontal and vertical), pro-saccade, and anti-saccade. To compare cognitive performance and eye movement parameters, t-tests or chi-square tests were appropriately used. Multi-variable regression analyses were used to estimate the risk of abnormal eye movement to aMCI.
Results: Compared to the CN group, the aMCI group had slower saccade velocity (p=0.016) in the anti-saccade task; significantly, they had greater total deviation (p < 0.05) and greater number of deviation (p < 0.01) (including horizontal and vertical). The great number of deviations in smooth pursuit tasks was the highest eye movement risk factor to aMCI after adjusting sex, age, and HAMD scores (horizontal smooth pursuit task, OR=1.07, CI,1.00~1.02; vertical smooth pursuit task, OR=1.09, CI, 1.03~1.15).
Conclusions: Patients with aMCI had poorer performance in eye movement in anti-saccades and smooth pursuit tasks than normal adults. The two tasks might be sensitive paradigm batteries for eye movements in aMCI.
Keywords: Amnestic mild cognitive impairment; Smooth pursuit tasks; Anti-saccade task; Number of deviation; Saccade velocity
Introduction
Over the past decades, life expectancy has been increasing rapidly worldwide. Age-related conditions, such as Alzheimer's Disease (AD) and other dementias, were the seventh leading cause of death in 2019 reported by the World Health Organization [1]. AD accounts for an estimated 60 - 80 % of dementia [2].
AD is a neurodegenerative disease characterized by memory loss and slowly progressive multiple cognitive decline with functional impact [3]. Mild Cognitive Impairment (MCI) has been considered the preclinical stage of AD. Based on clinical presentation, MCI can be categorized as amnestic MCI (aMCI) and non-amnestic MCI (naMCI) [4]. Evidence found structural differences between aMCI and naMCI in certain brain regions, such as the hippocampus and entorhinal cortex [5]. People with aMCI are at much greater risk of progressing to AD than naMCI, with an annual conversion rate of 5-17% [6]. To date, there are no effective treatments for AD, especially for the middle and late phases of the disease. Thus, it is urgent to distinguish early and accurately to delay or prevent the condition's onset.
Recently, various approaches, such as amyloid beta testing both in blood and cerebrospinal fluid [7,8], amyloid-Positron Emission Tomography (PET) imaging [9], biological markers, and neuroimaging, have been proposed for screening and identification of MCI due to AD. Biological markers may offer the most promising path to detect MCI before symptoms [7]. Eye Movements (EMs) are becoming popular biological markers due to the development of accurate, affordable, moveable, and easy-to-use eye trackers. In contemporary neuroscience, EMs are vital in understanding cognition and behavior. Several decades of research have demonstrated that eye tracking can provide a wealth of information for cognition. Fixation, smooth pursuit, and saccades are the most common paradigms in EMs assessed for AD [10,11].
Evidence suggests that the performance of EMs could inform on impaired cognition of AD. Compared to healthy older, AD patients make more incorrect saccades and fewer corrections after committing an error [12]. In general, in saccades and smooth pursuit tasks, there appears to be slow saccades velocity, increased latency, and increased frequency of antisaccade errors due to the disease [13-15]. With impaired visuospatial judgment, mild AD patients made more errors on a spatial decision task than controls [16]. Furthermore, the performance in antisaccade tasks appears to be correlated with neuropsychological test scores, such as MMSE, backward digit span, Stroop inhibition, and verbal fluency [14,17].
Eye movement deficits may develop in the early course of AD. In the antisaccade paradigm, patients with MCI had a smaller proportion of correct responses and a higher frequency of errors than health control [18]. A strong correlation has been reported between antisaccade error rate and cortical thinning in MCI [19]. Further, previous work has shown that saccade paradigms could distinguish MCI from controls [15]. Also, eye movement parameters were stable indicators to distinguish MCI, which were not affected by different testing versions [20]. Furthermore, the EMs performance is strongly correlated with the severity of AD [21].
Few studies have investigated eye movements in aMCI. However, MCI was a heterogeneous condition. It is necessary to focus on the aMCI sub-type, which has a higher conversation rate to AD. One recent research using the EyeLink Desktop eye-tracker found that the aMCI group had longer mean latency and a higher proportion of anti-saccade errors than the control group [22]. Moreover, Chehrehnegar N et al. suggested that saccadic were sensitive measures to distinguish aMCI from normal participants; even more, these parameters were strongly correlated with neuropsychological measures [23]. The study used pro-/anti-saccade tasks and gap/overgap saccade paradigm to record eye movement by a remote desktop eye tracker. However, the scarcity of eye movement studies in aMCI necessitates further research to determine the features and degree of eye movement change in aMCI in different areas and with differing apparatuses.
Previous research has created a strong foundation for understanding AD-related changes to eye movements, but there still needs to be answers that merit further investigation. This study aimed to investigate the eye movement features of patients with aMCI by a head-mounted intelligent analysis system in China, then to explore the risk of abnormal eye movement to aMCI, to provide new evidence for developing a standardized eye tracking test battery for early identification of AD.
Materials and Methods
Participants
This study is a cross-sectional study. Sixty-six participants were enrolled in the Memory Disorders Clinic of the Department of Neurology at the First People's Hospital of Foshan between August 2022 and December 2022. Nine cases were excluded because of the diagnosis of AD; Seventeen cases were excluded because the eye movements and neuropsychological test data were unqualified (Figure 1). Forty participants were included for analysis. Nineteen participants were diagnosed with aMCI. Twenty-one Cognitive Normal (CN) older adults with matching demographic information (age, sex, and education level). Demographic characteristics,medical history, and Hamilton Depression Scale (HAMD) scores were collected in face-to-face interviews. The inclusion criteria of the aMCI were detailed in our previous paper [24].
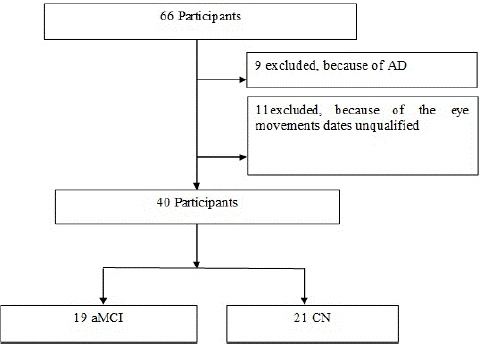
Figure 1: Flow Diagram of Participants.
Exclusion criteria for all participants are as follows: (1) illiteracy; (2) participants with cognitive decline caused by other diseases, such as a history of stroke, Parkinson's disease, brain injury, and brain surgery. (3) cataracts, glaucoma, or other eye diseases may affect eye movement tests. (4) other systemic conditions could have caused ocular symptoms affecting eye tracking. Ethics approval was obtained from the Research Ethics Board of the First People's Hospital of Foshan. All participants signed written consent forms upon enrollment.
Neuropsychological Assessment
A neuropsychological test battery was carried out, which included Mini-Mental State Examination (MMSE) [25], Clinical Dementia Rating (CDR) [26], Auditory Verbal Learning Test (AVLT), Boston Naming Test (BNT), Stroop Color-Word Test (SCWT), Symbol Digit Modalities Test (SDMT) and Clock Drawing Test (CDT), which were detailed in previous paper [24].
Eye movements Assessment
Eye movements were recorded using a head-mounted intelligent analysis and evaluation system, EyeKnow (Beijing CAS-Ruiyi Information Technology Co., Ltd.), which used a pupil center corneal reflections method that sampled at a 90-Hz rate. Participants were seated in a quiet room and wore the collection device on their heads. Before the experiment, a nine-point calibration procedure was performed to calibrate the eye movement data with a maximum calibration error of 2° in radius. The eye movement parameters were analyzed and calculated by the eye-tracking system's embedded data processing module.
① The assessment involved four tasks: fixation, smooth pursuit (horizontal and vertical), pro-saccade, and aFixation task: one test for sustained fixation on a static object. A stationary green target dot was presented, and participants were asked to fixate on the target dot stably as soon as possible. In the task, accuracy rate, total deviation (>4°), and number of deviations (>4°) were analyzed. Accuracy rate was defined as the proportion of time spent fixating on the spot relative to the duration of the entire fixation task. Total deviation was defined as the cumulative degree of deviation from the moving spot across the whole task. The number of deviations was defined as the total count of instances where the eye deviated by more than 4° from the moving spot throughout the fixation task.
② Pro-saccade task: In the pro-saccade task, a green target dot was presented at the center of the display and randomly shifted ±15° horizontally and vertically away from the center. Participants were asked to promptly and accurately gaze toward the target dot. The parameters of saccade latency, velocity, and amplitude were analyzed. The saccade latency was defined as the time for a saccade to begin after the target appeared. The saccade velocity was defined as the angular speed of the eye movement, calculated by measuring the angular displacement during the interval from saccade onset to saccade offset. The saccade amplitude was a key metric for assessing the range of eye movement, which was defined as the angle of saccade movement from central to target expressed in degrees.
③ Anti-saccade Task: Similar to the pro-saccade task, a green target dot was presented, but participants were asked to execute a saccade to the opposite position of the target dot as soon as possible. In the task, the analyzed parameters included saccade latency, velocity, and error correction reaction time. The saccade latency was the time duration between the target's onset and the corresponding saccade's initiation. The saccade velocity was the angular speed of the eye movement, calculated by measuring the angular displacement from saccade onset to saccade offset. The error correction reaction time was the duration from the onset of the corrective saccade to its completion, as it reached the opposite position.
④ Smooth pursuit tasks (including horizontal and vertical smooth pursuit): a test for sustained object tracking. During the tasks, a green dot moved along a sinusoidal trajectory with a horizontal/vertical amplitude of 20° and a frequency of 0.2 Hz. Participants were asked to track the sinusoidal movement of the target dot continuously. The analyzed parameters were pursuit accuracy, total deviation, and number of deviations. The pursuit accuracy was the ratio of the accurately tracked duration of the target dot to the entire task duration; participant gaze points within a 2◦ radius from the center of the target were considered accurately tracked. The total deviation (>4°) was the cumulative degree of deviation from the moving spot across the entire task. The number of deviations was the total count of instances where the eye deviated by more than 4° from the moving spot in the task.
Statistical Analysis
The comparison of demographic characteristics, scores of neuropsychological assessments, and eye movement parameters between the two groups was appropriate using a t-test or chi-square test. Multi-variable regression analyses were used to estimate the effect values (OR) and 95% Confidence Intervals (CI) to examine the risk or abnormal eye movement to aMCI. Age, sex, and HAMD scores were adjusted. A smooth curve was administered to describe the relationship between the two. Analyses were conducted by the statistical software packages R (http://www.R-project.org, The R Foundation) and Empower Stats (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). All the p-values <0.05 were considered statistically significant.
Results
Participants Characteristics
The flowchart in Fig 1 illustrates the process of sample selection.
The participants' demographic characteristics, neuropsychological, and eye movement performance were described in Table 1, Figure 2, and Figure 3. Compared to CN, participants with aMCI had poorer cognitive performance, including global cognitive function (p=0.003) and cognitive domains (memory, visuospatial skills, and executive function (p < 0.05). There are more males in the aMCI group. Variables of age, education, ADL, and HAMD did not differ between the two groups (p > 0.05).
Total (N=40)
P-value
Demographic characteristics
70.0 (3.7)
69.4 (4.3)
70.5 (2.8)
0.355
Sex, Male, n(%)
20 (50.0)
7 (33.3)
13 (68.4)
0.027*
Education, years, n(%)
0.89
Primary school
12 (30.0)
7 (33.3)
5 (26.3)
Middle school
20 (50.0)
10 (47.6)
10 (52.6)
High school and above
8 (20.0)
4 (19.0)
4 (21.0)
HAMD, scores,
6.50 (3.00-9.25)
8.00 (4.00-11.00)
5.00 (3.00-8.00)
0.196
Median (Q1-Q3)
MMSE, scores
26.5 ± 2.1
27.2 ± 1.4
25.7 ± 2.5
0.029*
AVLT immediate, scores
5.47 ± 2.04
6.05 ± 2.2
4.8 ± 1.6
0.061
AVLT 5 min, scores
6.7 ± 2.5
7.6 ± 2.4
5.6 ± 2.3
0.012*
CDT, scores
8.6 ± 1.9
9.4 ± 1.5
7.8 ± 2.1
0.010*
BNT, scores
22.3 ± 3.8
23.3± 3.3
21.3 ± 4.3
0.102
SDMT, scores
36.8 ± 9.7
38.7 ± 7.0
34.6 ± 11.9
0.192
Stroop Test A, scores
10.8 ± 2.5
9.8 ± 1.8
11.8 ± 2.7
0.011*
Stroop Test B, scores
17.8 ± 8.7
14.4 ± 4.7
21.6± 10.6
0.008**
Stroop Test C, scores
33.9 ± 16.0
30.7 ± 13.9
37.3± 17.8
0.197
20.5 (1.0)
21.2 (1.5)
0.077
Eye movements assessment
81.41 (16.49)
0.649
17.00 (10.75-23.25)
0.881
168.49 (81.85)
0.961
326.99 (55.76)
0.452
234.92 (64.32)
0.188
13.29 (0.75)
0.951
403.24 (92.59)
0.832
0.49
232.33 (69.71)
0.016*
71.59 (22.56)
0.232
Horizontal _Total Deviation, (°), Median (Q1-Q3)
176.98 (109.62-247.50)
0.020*
32.00 (20.00-48.50)
0.005**
63.15 (17.98)
0.18
191.29 (130.34-280.41)
0.012*
38.00 (22.75-52.00)
0.001**
SD, Standard Deviations; (Q1-Q3), (25% quantile - 75% quantile); ADL, Activities of Daily Living; MMSE, Mini-mental state examination continuous; AVLT, Auditory Verbal Learning Test; CDT, Clock Draw Test; BNT, Boston Naming Test; SDMT, Symbol Digit Modalities Test. **P < 0.01; * P < 0.05.
Table 1: Demographic and clinical characteristics of the participants.
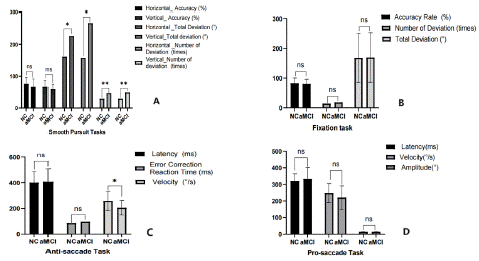
Figure 2: The difference of eye movement performance between aMCI and CN. A) Smooth Pursuit Tasks (included horizontal and vertical smooth pursuit); B) Fixation Task; C) Anti-saccade Task; D) Pro-saccade task.
(**P < 0.01; *P < 0.05)
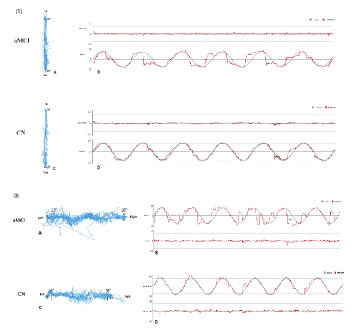
Figure 3: Visual evaluation of the smooth pursuit tasks [vertical (1) and horizontal (2)] in the aMCI and CN. (1)-A, (2)-A, (1)-C, (2)-C: Plotted planar gaze trajectories (vertical and horizontal) in the tasks; (1)-B, (2)-B, (1)-D, (2)-D: spatio-temporal plots of eye movement trajectories during the task.(**P < 0.01; *P < 0.05)
Table 1 presents the results of the eye movement tests. The aMCI group had slower saccade velocity (p=0.016) in the anti-saccade task and a significantly greater total deviation (p < 0.05) and greater number of deviations (p < 0.01) in smooth pursuit tasks (including horizontal and vertical) compared to the CN group. Despite no statistical differences, the aMCI group had longer saccade latency and lower saccade velocity in pro-saccade, longer saccade latency and error correction reaction time in anti-saccade tasks, and less pursuit accuracy in smooth pursuit tasks. The visual evaluation of the smooth pursuit tasks, including planar gaze trajectories and spatiotemporal plots of eye movement trajectories during the task, were plotted in Fig 3. The performance of aMCI patients was worse than that of CN. However, in the fixation and pro-saccade tasks, the eye movement parameters show no difference between the two groups.
Association of Eye Movements and Cognitive Function
Figure 4 plotted the smooth curve of eye movement performance and aMCI, which showed that the great number of deviations in smooth pursuit tasks (both horizontal and vertical) and the slow saccade velocity (both in the anti-saccade task and pro-saccade task) were associated with the risk of aMCI. The multi-variable regression analyses the extent of the risk of abnormal eye movements to aMCI. After adjusted sex, age, and HAMD scores, the great number of deviations in smooth pursuit tasks were the highest indicators of aMCI risk (horizontal smooth pursuit task, OR=1.07, CI,1.00~1.02; vertical smooth pursuit task, OR=1.09, CI, 1.03~1.15). The great total deviation in the smooth pursuit task and the slow saccade velocity of the anti-saccade task increased the aMCI risk as well (OR=1.01 and OR= 0.99) (Table 2).
Non-adjusted
Adjust I
Adjust II
OR (95% CI) P value
Saccade Velocity of Pro-saccade task
0.99 (0.98, 1.00) 0.191
0.99 (0.98, 1.00) 0.076
0.99 (0.98, 1.00) 0.095
Saccade Velocity of Anti-saccade task
0.99 (0.98, 1.00) 0.028
0.99 (0.97, 1.00) 0.023
0.99 (0.97, 1.00) 0.030*
Horizontal _Total Deviation
1.01 (1.00, 1.02) 0.033
1.01 (1.00, 1.02) 0.025
1.01 (1.00, 1.02) 0.019*
Horizontal _Number of Deviation
1.06 (1.01, 1.10) 0.014
1.07 (1.01, 1.13) 0.015
1.07 (1.02, 1.14) 0.012*
Vertical_Total deviation
1.01 (1.00, 1.01) 0.025
1.01 (1.00, 1.02) 0.013
1.01 (1.00, 1.02) 0.015*
Vertical_Number of deviation
1.07 (1.02, 1.13) 0.006
1.09 (1.03, 1.15) 0.004
1.09 (1.03, 1.15) 0.004**
Non-adjusted model adjusts for: None; Adjust I model adjust for: sex; age; Adjust II model adjust for: sex; age; HAMD scores.; **P < 0.01; * P < 0.05.
Table 2: Association of eye movements performance with aMCI.
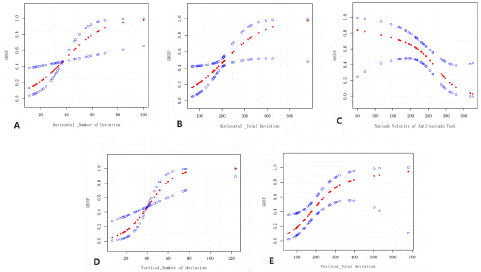
Figure 4: The smooth curve of the association between eye movements and aMCI. A) Vertical_Number of deviations in smooth pursuit task, B) Vertical_Total deviation in smooth pursuit task, C) Saccade Velocity in Anti-saccade task, D) Vertical_Number of deviations in smooth pursuit task, E. Vertical_Total deviation in smooth pursuit task.)
Discussion
The present study found that, aMCI individuals had poorer cognitive performance, especially in global cognitive function, memory, visuospatial skills, and executive function. Based on a head-mounted intelligence analysis system, the aMCI individuals' eye movement features were slower saccade velocity in anti-saccade tasks, greater total deviation, and greater number of deviations in smooth pursuit tasks. Moreover, the great number of deviations in smooth pursuit tasks (horizontal and vertical) and the slow saccade velocity (both in the anti-saccade and pro-saccade tasks) increased the risk of aMCI. Consequently, EMs may provide an indirect link to neuronal and cognitive functioning.
One of the key results of our study was the aMCI individuals had slower saccade velocity in antisaccade tasks, greater total deviations and greater the number of deviations in smooth pursuit tasks than health controls. A review pointed out that the eyes share many neural and vascular similarities to the brain and numerous cortical or subcortical regions, which are affected by cognitive impairment in the triggering and regulation of Ems [27]. The network involved in the saccades paradigm includes subcortical and cortical regions [27,28]. AD pathology research found that 52% of the cognitively intact subjects, and all subjects with MCI or dementia, had dense neurofibrillary tangles, neuropil threads, and tau-immunoreactive neurites surrounding neuritic plaques in visual association cortex [29]. AD-related changes developed impairments of inhibitory control and eye movement error correction [30], and were the fundamental cause of eye movement impairment, especially in saccades and smooth pursuit motion [13].
Anti-saccade is a voluntary saccades paradigm resulting from purposeful activity, in which more extensive reliance on higher-level executive control and increasingly complex brain stimulation patterns. Evidence from Functional Magnetic Resonance Imaging (fMRI) data revealed that aMCI showed reduced activation in frontal eye fields and increased inhibitory errors when performing the anti-saccade task [31]. In the eye movement task, inhibition of the reflexive response for a visually guided saccade to the target and reversing the stimulus location into a voluntary motor command to look in the opposite direction of the stimulus. Anti-saccade trials activate the oculomotor network and may also recruit additional brain areas. The anti-saccade task encompasses various cognitive processes, such as decision-making, working memory [32-34], goal-oriented behavior, and attention [35]. In our study, the cognitive function, especially memory, visuospatial skills, and executive function, were significantly damaged in aMCI, which might provide an intimate link between neurodegeneration and abnormal eye movements. That is, the abnormal eye movements might infer AD-related changes in cognitive processing caused by degenerative brain disorders. Meanwhile, saccade velocity in the anti-saccade task was a sensitive parameter.
In smooth pursuit tasks, a participant must continuously attempt to follow and hold their gaze on a moving target. This study's target starts from left to right (horizontal pursuit) and up to down (vertical pursuit). Smooth pursuit is implemented by a continuous feedback loop in the brain that undergoes correction throughout the pursuit process [36]. Motion information from the moving target is extracted by the lateral occipitotemporal cortex, which sends signals to a pursuit-specific portion [37]. Then, the signal continues to brainstem regions that subserve saccade generation and send the final motor commands to move the eyes [38]. Previous research has provided some evidence of the smooth pursuit impairment of patients with AD. While tracking the target, the disorders of smooth pursuit, such as large-amplitude saccadic intrusions, lower initial acceleration, decreased velocity, more compensatory saccades, and more disrupted pursuit in the direction of target motion, were found in AD [39-41]. One recent study in Chinese showed that cognitive impairment participants exhibited disorganized trajectories in smooth pursuit tasks, which suggested less stable and coordinated eye movements [42].
Notably, we focused on smooth pursuit permanence in aMCI individuals in this study. We found that the total deviation and the number of deviations were greater than the CN group, consistent with previous studies in AD or MCI. Further, the great number of deviations in smooth pursuit tasks were the highest indicators of aMCI risk (horizontal smooth pursuit task, OR=1.07, CI,1.00~1.02; vertical smooth pursuit task, OR=1.09, CI, 1.03~1.15). The risk was higher than the saccade velocity of the anti-saccade task (OR= 0.99). Therefore, smooth pursuit is another sensitive and vital task for aMCI.
Several limitations should be acknowledged. First, this study is a cross-sectional design with its inherent deficiency. A follow-up examination is necessary to reveal the risk of abnormal eye movement to aMCI in depth. Second, as the sex of the two groups had differences, when we estimated the effect values to examine the extent of the association between the abnormal eye movement and aMCI in multi-variable regression analyses, sex was adjusted. Third, the head-mounted intelligent analysis system is much smaller than the desktop eye-tracker, so the patients with severe cognitive impairment could not have accomplished the task better. Overall, based on a head-mounted intelligent analysis system, we preliminary found that the eye movement features of aMCI were: 1) Slow saccade velocity anti-saccades. 2) Great total deviation in smooth pursuit tasks. 3)High number of deviations in smooth pursuit tasks. Both anti-saccade and smooth pursuit might be sensitive tasks to detect aMCI. These results may help develop standardized test batteries of eye movements to distinguish aMCI from normal older adults accurately, sensitively, affordably, and movably. Further studies are needed to validate the results with larger sample sizes and more populations.
Conclusion
AMCI individuals had poor performance in eye movement in anti-saccades and smooth pursuit tasks. The number of deviations in the smooth pursuit task and saccade velocity in the anti-saccade task were effective indicators for aMCI. Both anti-saccade and smooth pursuit tasks might be sensitive tasks to detect aMCI.
Author Statements
Competing Interests
All authors have seen the manuscript and approved to submit to your journal.
Acknowledgments
We thank all participants in this study. We want to thank the team of the First People's Hospital of Foshan, who administered the cognitive evaluation and the eye movement monitoring.
Fundings
This work was supported by the National Key R&D Program of China (Grant No. 2018YFC2001700), the Special Fund of the Foshan Summit plan (Grant No. 2019A011), and the FoshanScience and Technology Bureau Project (Grant No.1920001001161).
CRediT authorship contribution statement
Hongzhen Zhou and Haiqun Xie: Conceptualization, Project administration; Shuyun Huang and Yajing Liu, Methodology, writing review & editing; Xia Xiang, Pan Shang, and Chunyan Zhang: Investigation, Data curation; Meiqiu Li and Jiali Luo: Validation, Formal analysis; Xiaoying Zhong: Investigation and Formal analysis; Yun Huang:Writing original draft.
Data Availability
Data will be made available on request.nti-saccade.
References
- World Health Organization. Global Health Estimates: Life expectancy and leading causes of death and disability. 2020.
- 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023; 19: 1598-1695.
- Musoke P, Olum R, Kembabazi S, Nantaayi B, Bongomin F, Kaddumukasa M, et al. Assessment of the Knowledge and Attitude Towards Dementia Among Undergraduate University Students in Uganda. Adv Med Educ Pract. 2021; 12: 635-646.
- Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013; 29: 753-772.
- Csukly G, Siraly E, Fodor Z, Horvath A, Salacz P, Hidasi Z, et al. The Differentiation of Amnestic Type MCI from the Non-Amnestic Types by Structural MRI. Front Aging Neurosci. 2016; 8: 52.
- Cheng YW, Chen TF, Chiu MJ. From mild cognitive impairment to subjective cognitive decline: conceptual and methodological evolution. Neuropsychiatr Dis Treat. 2017; 13: 491-498.
- Jack CJ, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018; 14: 535-562.
- Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, et al. High-performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature. 2018; 554: 249-254.
- Hameed S, Fuh JL, Senanarong V, Ebenezer EGM, Looi I, Dominguez JC, et al. Role of Fluid Biomarkers and PET Imaging in Early Diagnosis and its Clinical Implication in the Management of Alzheimer’s Disease. J Alzheimers Dis Rep. 2020; 4: 21-37.
- Garbutt S, Matlin A, Hellmuth J, Schenk AK, Jhonson JK, Rosen H, et al. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer’s disease. Brain. 2008; 131: 1268-1281.
- Pavisic IM, Firth NC, Parsons S, Rego DM, Shakespeare TJ, et al. Eyetracking Metrics in Young Onset Alzheimer’s Disease: A Window into Cognitive Visual Functions. Front Neurol. 2017; 8: 377.
- Crawford TJ, Higham S, Renvoize T, Patel J, Dale M, Suriya A, et al. Inhibitory control of saccadic eye movements and cognitive impairment in Alzheimer’s disease. Biol Psychiatry. 2005; 57: 1052-1060.
- Robert J. Molitor PC, Ally BA. Eye Movements in Alzheimer’s Disease. J Alzheimers Dis, 2015; 44: 1-12.
- Currie J, Ramsden B, McArthur C, Maruff P. Validation of a clinical antisaccadic eye movement test in the assessment of dementia. Arch Neurol. 1991; 48: 644-648.
- Opwonya J, Doan D, Kim SG, Kim JL, Ku B, Kim S, et al. Saccadic Eye Movement in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neuropsychol Rev. 2022; 32: 193-227.
- Laurens B, Planche V, Cubizolle S, Declerck L, Dupouy S, Formaglio M, et al. A Spatial Decision Eye-Tracking Task in Patients with Prodromal and Mild Alzheimer’s Disease. J Alzheimers Dis. 2019; 71: 613-621.
- Abel LA, Unverzagt F, Yee RD. Effects of stimulus predictability and interstimulus gap on saccades in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002; 13: 235-243.
- Opwonya J, Wang C, Jiang KM, Lee K, Kim J, Kim JU. Inhibitory Control of Saccadic Eye Movements and Cognitive Impairment in Mild Cognitive Impairment. Front Aging Neurosci. 2022; 14: 871432.
- Heuer HW, Mirsky JB, Kong EL, Dickerson BC, Miller BL, Kramer JH, et al. Antisaccade task reflects cortical involvement in mild cognitive impairment. Neurology. 2013; 81: 1235-1243.
- Nie J, Qiu Q, Phillips M, Sun L, Yan F, Lin X, et al. Early Diagnosis of Mild Cognitive Impairment Based on Eye Movement Parameters in an Aging Chinese population. Front Aging Neurosci. 2020; 12: 221.
- Chehrehnegar N, Shati M, Esmaeili M, Foroughan M. Executive function deficits in mild cognitive impairment: evidence from saccade tasks. Aging Ment Health. 2022; 26: 1001-1009.
- Wilcockson TDW, Mardanbegi D, Xia B, Taylor S, Sawyer P, Gellersen HW, et al. Abnormalities of saccadic eye movements in dementia due to Alzheimer’s disease and mild cognitive impairment. Aging (Albany, NY.). 2019; 11: 5389-5398.
- Chehrehnegar N, Nejati V, Shati M, Esmaeili M, Rezvani Z, Gaghi M, et al. Behavioral and cognitive markers of mild cognitive impairment: diagnostic value of saccadic eye movements and Simon task. Aging Clin Exp Res. 2019; 31: 1591-1600.
- Huang S, Zhou X, Liu Y, Luo Z, Lv Z, Shang P, et al. High Fall Risk Associated With Memory Deficit and Brain Lobes Atrophy Among Elderly with Amnestic Mild Cognitive Impairment and Mild Alzheimer’s Disease. Front Neurosci. 2022; 16: 896437.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12: 189-198.
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993; 43: 2412-2414.
- Pierrot-Deseilligny C, Milea D, Muri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol, 2004; 17: 17-25.
- Leigh RJ, Kennard C. Using saccades as a research tool in the clinical neurosciences. Brain. 2004; 127: 460-477.
- McKee AC, Au R, Cabral HJ, Kowall NW, Seshadri S, Kubilus CA, et al. Visual association pathology in preclinical Alzheimer disease. J Neuropathol Exp Neurol. 2006; 65: 621-630.
- Crawford TJ, Higham S, Mayes J, Dale M, Shaunak S, Lekwuwa G, et al. The role of working memory and attentional disengagement on inhibitory control: effects of aging and Alzheimer’s disease. Age (Dordr). 2013; 35: 1637-1650.
- Alichniewicz KK, Brunner F, Klunemann HH, Greenlee MW. Neural correlates of saccadic inhibition in healthy elderly and patients with amnestic mild cognitive impairment. Front Psychol. 2013; 4: 467.
- Everling S, Johnston K. Control of the superior colliculus by the lateral prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 2013; 368: 20130068.
- Baddeley AD, Bressi S, Della SS, Logie R, Spinnler H. The decline of working memory in Alzheimer’s disease. A longitudinal study. Brain. 1991; 114: 2521-2542.
- Parra M A, Granada J, Fernandez G. Memory-driven eye movements prospectively predict dementia in people at risk of Alzheimer’s disease. Alzheimers Dement (Amst). 2022; 14: e12386.
- Jamadar SD, Fielding J, Egan GF. Quantitative meta-analysis of fMRI and PET studies reveals consistent activation in fronto-striatal-parietal regions and cerebellum during antisaccades and prosaccades. Front Psychol. 2013; 4: 749.
- Krauzlis R J. Recasting the smooth pursuit eye movement system[J]. J Neurophysiol, 2004,91(2):591-603.
- Petit L, Haxby JV. Functional anatomy of pursuit eye movements in humans as revealed by fMRI. J Neurophysiol. 1999; 82: 463-471.
- Dicke PW, Barash S, Ilg UJ, Thier P. Single-neuron evidence for a contribution of the dorsal pontine nuclei to both types of target-directed eye movements, saccades and smooth-pursuit. Eur J Neurosci. 2004; 19: 609-624.
- Fletcher WA, Sharpe JA. Smooth pursuit dysfunction in Alzheimer’s disease. Neurology. 1988; 38: 272-277.
- Zaccara G, Gangemi PF, Muscas GC, Paganini M, Pallanti S, Parigi A, et al. Smooth-pursuit eye movements: alterations in Alzheimer’s disease. J Neurol Sci. 1992; 112: 81-89.
- Kuskowski MA, Malone SM, Mortimer JA, Dysken MW. Smooth pursuit eye movements in dementia of the Alzheimer type. Alzheimer Dis Assoc Disord. 1989; 3: 157-171.
- Lin J, Xu T, Yang X, Yang Q, Zhu Y, Wan M, et al. A detection model of cognitive impairment via the integrated gait and eye movement analysis from a large Chinese community cohort. Alzheimers Dement (Amst), 2024; 20: 1089-1101.