
Case Report
Austin J Clin Neurol. 2024; 11(2): 1168.
Case Report: Delayed Cerebral Ischemia with Infarction following an Undiagnosed Aneurysmal Rupture - The Diagnostic and Management Challenge
Gulia K¹; Doustmohammadi D¹; Ezzati S¹; Chang J²; Yang C¹; Lui F¹*
1College of Medicine, California Northstate University, USA
2Department of Neurology, Kaiser Permanente South Sacramento Medical Center, USA
*Corresponding author: Lui F, College of Medicine, California Northstate University, USA. Email: Forshing.Lui@cnsu.edu
Received: October 18, 2024; Accepted: November 06 2024 Published: November 13, 2024
Abstract
Delayed Cerebral Ischemia (DCI) typically occurs a few days after a patient suffers subarachnoid hemorrhage and can lead to a range of poor outcomes. It is primarily driven by vasospasm and its delayed and variable presentation complicates diagnosis and treatment. Here we report a case of DCI in a 31-yearold man who presented with acute onset aphasia, right-sided weakness, and difficulty swallowing. He had experienced a severe headache accompanied by nausea and vomiting 5 days prior, with an initial NIH stroke scale score of 10. Imaging revealed a left internal carotid artery aneurysm and a watershed infarct between the anterior cerebral artery and middle cerebral artery. The patient was treated with aneurysmal coiling, intraarterial verapamil, and oral nimodipine for suspected DCI secondary to subarachnoid hemorrhage. Close monitoring of the patient and treatment led to the resolution of neurological deficits over the subsequent days., with imaging confirming reperfusion. This case presents a unique challenge due to the delayed presentation and vague history. Diagnosis and treatment of DCI following aneurysmal rupture are complicated due to its delayed and variable onset. It is crucial to consider acute aneurysmal subarachnoid hemorrhage and DCI when evaluating patients who present with multiple stroke-like symptoms over several days as the management and prognosis differ from other cases of acute ischemic stroke.
Keywords: Delayed cerebral ischemia; Subarachnoid hemorrhage; Atypical presentation; Aneurysmal rupture; Cerebral vasospasm; Imaging modalities; Stroke
Introduction
Acute Subarachnoid Hemorrhages (SAH) have high mortality and morbidity and most survivors retain secondary deficits. Delayed Cerebral Ischemia (DCI), one important cause of neurological deficits secondary to SAHs, does not occur until days after initial bleeding [1,2]. While vasospasm is the primary cause of DCI, vasospasm prophylaxis alone does not improve outcomes in patients [3-5]. This highlights the multifactorial nature of DCI, as it can be attributed to microthromboemoblisms, loss of cerebral autoregulation, genetic polymorphisms, inflammation, and angiographic vasospasm [2,6,7].
DCI typically occurs 4-7 days after initial SAH but can occur from 3-14 days afterward. Due to its delayed onset, patients must be examined closely after a SAH despite sedation or ventilation [1,2,7,8]. DCI is characterized by focal neurological impairment or a decrease of 2 points on the Glasgow Coma Scale for longer than 1 hour [1,9]. Digital subtraction angiography is the gold standard for imaging suspected patients [10,11]. The delayed onset, presentation, and variable radiological findings of DCI have led to a need for thorough research and documentation of outcomes [9,12].
Prevention and treatment of DCI is complex, involving oral nimodipine to prevent vasospasm along with regular monitoring of the patient's blood pressure and volume status, as most patients with DCI have unstable blood pressure [13-17]. This case describes a patient presenting with a vague history who was found to have vasospasm-induced DCI after an undiagnosed SAH.
Case Presentation
A 31-year-old Punjabi male with no prior medical history presented to the ED with 2-3 days of progressive weakness and aphasia. History was obtained from patient's friend and sister due to patient’s language deficits. Five days prior to the ED visit, the patient experienced a severe headache while riding in a truck, associated with nausea and vomiting. He had no previous history of headaches, photophobia, hyperacusis, or fever. His friend recommended he go to the ED but the patient refused and took acetaminophen instead. His symptoms worsened, leading to difficulty with talking and swallowing. The following day he became non-verbal and was sent to the ED.
Hospital Course
On examination, patient was afebrile, with blood pressure of 116/83 and pulse of 56 bpm. There was no neck rigidity and Kernig’s sign was negative. He was alert but aphasic, uttering few unintelligible words, photophobic, and showed mild right face and arm weakness and right pronator drift. His NIH stroke scale was 10. Neurologic exam was otherwise unremarkable. Electrocardiogram showed normal sinus rhythm. Laboratory findings are shown in Table 1.
Lab Results
CMP
Value
Reference Value
Creatinine (mg/dL)
0.64
0.7 - 1.3
BUN (mg/dL)
8
8 - 20
Potassium (mEq/L)
4.1
3.5 - 5.0
Sodium (mEq/L)
137
136 - 145
Chloride (mEq/L)
105
96 - 106
CO2 (mEq/L)
26
23 - 30
CBC
Value
Reference Value
WBC Count (k/mcL)
9.8
4.0 - 11
Hgb (g/dL)
13.5
14 - 18
Hematocrit (%)
37.2
42 - 50
Platelet count (/uL)
224,000
150,000 - 140,000
PT INR
1.0
0.8 - 1.1
Table 1: Results of patient’s blood tests.
Head Computed Tomography (CT) (Figure 1) showed acute left temporal region hemorrhage with surrounding vasogenic edema in the left frontal lobe. Neurology was consulted, considering infectious or hemorrhagic encephalitis, vascular anomaly, and neoplasm as differential diagnoses . Acyclovir was started for empiric herpes simplex virus coverage. The patient could not consent to lumbar puncture, which was not performed.
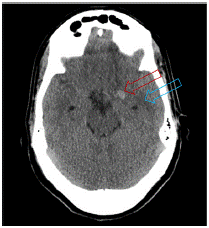
Figure 1: Head CT without contrast. Hyperdense lesion in the medial
temporal area lobe and hypodense surrounding area suggestive of edema.
Diffusion-Weighted (DWI) Magnetic Resonance Imaging (MRI) (Figure 2) of the brain showed acute infarcts in the watershed zone between the left Anterior Cerebral Artery (ACA) and Middle Cerebral Artery (MCA) territories, age-indeterminate high-grade stenosis of the paraclinoid left internal carotid artery with collateral formation involving the left ACA and MCA territories as well as a 1.3 cm aneurysm involving the left internal carotid artery (ICA) terminus. MRI showed no signs of acute intraparenchymal hemorrhage, mass effect, or herniation, but revealed subtle evidence of subarachnoid blood over the left cerebral peduncle and tentorium cerebelli. Physical examination findings of photophobia, expressive aphasia, and right hemiparesis 5 days after severe headache led to the diagnosis of acute subarachnoid sentinel bleed from the left ICA terminus aneurysm followed by delayed vasospasm and watershed infarction.
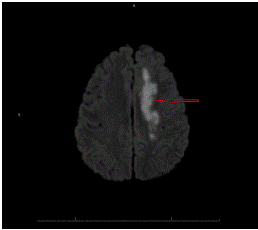
Figure 2: MRI. Acute infarcts in the watershed zone between the left ACA and MCA territories.
For higher spatial resolution, MR angiography (Figure 3) and CT angiography (Figure 4) of head and neck (Figure 3) were performed, confirming age-indeterminate high-grade severe stenosis of the paraclinoid left internal carotid artery, 7x9x7 mm aneurysm involving the left ICA terminus, additional adjacent medially enlarged left ICA measuring 4x7 mm APxTV, moderate diffuse narrowing of left M1 segment, and moderate-severe narrowing of right terminal ICA. Findings were confirmed by DSA (Figure 5).
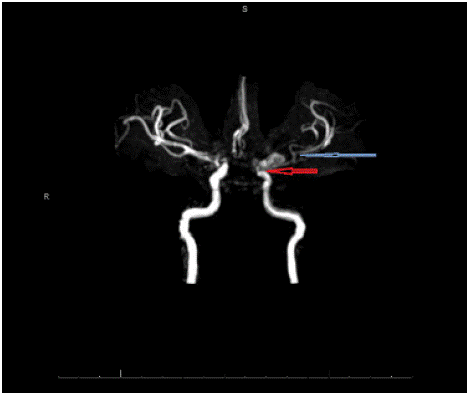
Figure 3: MR angiography. 1.3 cm aneurysm involving the left ICA terminus
and severe stenosis of left ICA (red arrow) and MCA (blue arrow).
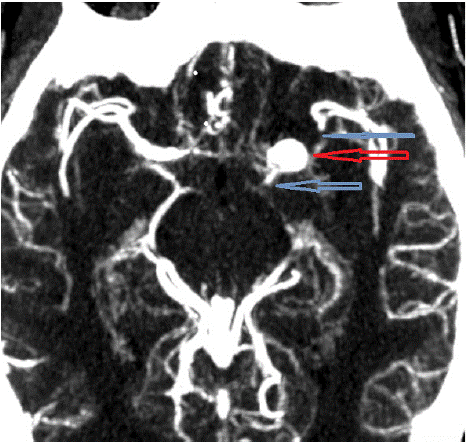
Figure 4: CTA. Left ICA aneurysm (red arrow) and stenosis of left ICA and
MCA (blue arrows).
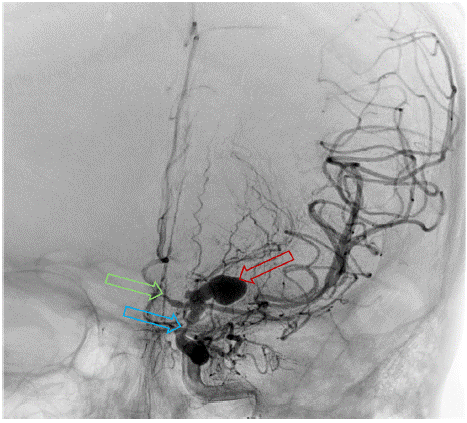
Figure 5: DSA. Stenosis of the left ICA (Blue) and ACA (Green), and left
ICA terminus aneurysm (Red).
Treatment and Outcome
Patient was transferred to the ICU for a ruptured left ICA aneurysm. He underwent aneurysmal coiling and received Intra- Arterial (IA) verapamil with hourly neurologic exams, as well as nimodipine 60 mg PO every 4 hours. Postoperatively, the patient's aphasia, pronator drift, and leg strength improved. The following day, the patient remained stable. 2 days after aneurysmal coiling, CTA showed improved bilateral supraclinoid ICA and left ACA spasm but unchanged right proximal ACA spasm.
Discussion
The Atypical Presentation of Our Case and the Diagnostic Challenge
This case presents a 31-year-old male with DCI following an undiagnosed SAH. SAH typically presents with a thunderclap headache, a sudden and severe headache often accompanied by nausea, vomiting, and photophobia [21]. However, the atypical progression of the patient’s symptoms complicated the clinical picture, leading to considerations of infectious encephalitis or vascular anomalies, rather than SAH with vasospasm. While atypical onset is uncommon, it can complicate the clinical course and it carries a risk of rebleeding due to misdiagnosis or delayed diagnosis [22]. This highlights a significant diagnostic challenge as DCI and vasospasm following SAH can manifest with subtle, variable symptoms that may not directly suggest the underlying etiology. The diagnostic approach involved multiple imaging modalities and was ultimately confirmed with MRI and CT. The use of imaging was important for identifying vascular pathology and ruling out other causes, underscoring the importance of thorough imaging evaluation in cases of unexplained neurological deficits following a headache [23].
Pathophysiology of Delayed Cerebral Ischemia Due to Vasospasm After Acute SAH
DCI is a multifactorial and serious complication of SAH contributing to morbidity and mortality [24,25]. Vasospasm is one of the primary causes of DCI and typically begins 3-4 days after aneurysm rupture, peaking around days 7-10 [26]. Vasospasminduced DCI may involve other mechanisms, including vascular dysfunction, impaired cerebral autoregulation, and inflammation.
As blood enters the subarachnoid space, there is an acute rise in intracranial pressure and a decrease in cerebral perfusion pressure, resulting in global ischemia closely followed by the “catecholamine surge,” an increase in sympathetic activity which can persist for several days [27,28]. The sympathetic activity reflects the severity of SAH and is closely related to the development of delayed vasospasm [29]. Transient global ischemia causes endothelial injury and disrupts the Blood Brain Barrier (BBB), which further contributes to vascular dysfunction. Within 24 hours of SAH, endothelial cells undergo apoptosis, leading to breakdown of the BBB and exposure of subendothelial collagen, promoting coagulation and microthrombi formation [30].
Cerebral autoregulation maintains cerebral blood flow in response to changes in blood pressure. The presence of blood in the subarachnoid space and vasospasm of large arteries can disrupt the autoregulatory function of cerebral vessels, making them less responsive to changes in systemic blood pressure and prone to ischemia [31]. This impairment in cerebrovascular autoregulation is associated with vasospasm and DCI in SAH [32,33].
Release of vasoactive substances and inflammatory mediators also contributes to development of DCI. Hypoxia from transient ischemia triggers expression of endothelin-1, a potent vasoconstrictor [34]. Hemoglobin breakdown products, oxyhemoglobin and deoxyhemoglobin, increase in the subarachnoid space during the development of DCI which can stimulate endothelin production and cause vasoconstriction through the Rho/Rho-kinase and protein kinase C pathways [35-37]. Additionally, oxyhemoglobin scavenges nitric oxide (NO), reducing availability. This leads to NOS uncoupling, where NO production is disrupted and superoxide is produced, causing oxidative stress and exacerbating vascular dysfunction [38,39].
Clinical Significance of Vasospasm
In this case, vasospasm led to significant cerebral ischemia and neurological deficits, including aphasia and right-sided weakness. Cerebral vasospasm reduces perfusion to the brain, causing ischemia or infarction in downstream territories [40]. This is concerning in SAH, where risk of vasospasm and subsequent DCI can persist for two weeks after initial bleed, demonstrating the importance of close monitoring and intensive management to prevent DCI, even in patients with atypical presentation [41,42].
Treatment and Management
The treatment approach included aneurysm coiling to secure the source of bleeding and administration of IA verapamil to relieve vasospasm. Verapamil targets constricted arteries, causing local vasodilation and improving cerebral blood flow [43,44]. Oral nimodipine was administered every four hours, as it has been shown to reduce occurrence of neurological deficits due to vasospasm [45]. Nimodipine has high lipid solubility, passes through the BBB, and exerts neuroprotective effects through reduction of free radical formation and attenuation of endothelin neurotoxicity [46]. Thus, it maintains cerebral blood flow despite vasospasm. This combined approach of endovascular therapy and pharmacological management led to stabilization of the patient and improvement of his symptoms.
Patient's Recovery and Outcome
Following these interventions, the patient showed good neurological recovery. These outcomes reflect the effectiveness of early diagnosis, appropriate use of imaging, and targeted therapy in managing vasospasm-induced DCI. Regular neurological checks and repeat imaging were essential in monitoring progress and guiding treatment adjustments, demonstrating the need for continuous vigilance in the post-SAH period [47,48].
Educational Value and Lessons Learned
Our case is unusual because the patient presented clinically with a 5-day history of headache, nausea, and vomiting followed by evolving aphasia and right hemiparesis. Without a typical history suggestive of acute SAH, there are several differential diagnoses including acute encephalitis and other lesions affecting the left hemisphere. His head CT at the ED showed a small hemorrhage with surrounding edema. This is the best clue to an underlying vascular lesion such as a bleeding aneurysm. Subsequent urgent MRI confirmed the watershed infarction between the left ACA and MCA due to underlying vasospasm, stenosis, and DCI. Immediate treatment with endovascular coiling of the aneurysm, IA verapamil and oral nimodipine, together with close ICU monitoring resulted in a good outcome for our patient. Our case demonstrates the importance of recognizing DCI due to vasospasm after an undiagnosed acute SAH as an important cause of acute onset headache with evolving focal neurological deficits over a few days. Prompt recognition of the diagnosis with modern imaging techniques and treatment is of utmost importance in improving patient outcomes. A multispecialty collaborative team approach involving the emergency physician, neurologist, neuroradiologist, neurointerventionist, neurosurgeon, and neurointensivist is important in improving patient outcomes.
Conclusion
This case highlights the challenges of diagnosing and managing delayed cerebral ischemia due to vasospasm following acute subarachnoid hemorrhage. The atypical presentation and delayed diagnosis underscore the need for vigilance in monitoring for vasospasm, even in patients exhibiting delayed or non-characteristic symptoms. Early intervention is guided by imaging and includes aneurysm coiling and targeted pharmacological therapies, which effectively resolved the patient’s symptoms and prevented long-term neurological damage. The insights gained from this case can help inform future guidelines for the management of DCI post-SAH, particularly emphasizing the importance of early and aggressive treatment to improve patient outcomes.
References
- Abdulazim A, Heilig M, Rinkel G, Etminan N. Diagnosis of delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage and rriggers for intervention. Neurocrit Care. 2023; 39: 311-319.
- Yamaki VN, Cavalcanti DD, Figueiredo EG. Delayed ischemic neurologic deficit after aneurysmal subarachnoid hemorrhage. Asian J Neurosurg. 2019;14: 641-647.
- Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS- 1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008; 39: 3015-3021.
- Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, Vajkoczy P, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011; 10: 618-625.
- Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, et al. Randomised trial of clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping (CONSCIOUS-2). Acta Neurochir Suppl. 2013; 115: 27-31.
- Budohoski KP, Guilfoyle M, Helmy A, Huuskonen T, Czosnyka M, Kirollos R, et al. The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2014; 85: 1343-1353.
- Francoeur CL, Mayer SA. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care. 2016; 20: 277.
- Crobeddu E, Mittal MK, Dupont S, Wijdicks EFM, Lanzino G, Rabinstein AA. Predicting the lack of development of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke. 2012; 43: 687- 701.
- Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010; 41: 2391-2395.
- Wilson CD, Shankar JJS. Diagnosing vasospasm after subarachnoid hemorrhage. CTA and CTP. Can J Neurol Sci. 2014; 41: 314-319.
- Connolly Jr ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012; 43: 1711-1737.
- Goursaud S, Martinez de Lizarrondo S, Grolleau F, Chagnot A, Agin V, Maubert E, et al. Delayed cerebral ischemia after subarachnoid hemorrhage: Is there a relevant experimental model? A systematic review of preclinical literature. Front. Cardiovasc. Med. 2021; 8: 752769.
- Eren F, Yildogan AT, Demir A, Ozguncu C, Yilmaz SE. Delayed cerebral ischemia and therapeutic approaches after subarachnoid hemorrhage. Explor Neuroprot Ther. 2022; 2: 162-173.
- Chugh C, Agarwal H. Cerebral vasospasm and delayed cerebral ischemia: Review of literature and the management approach. Neurol India. 2019; 67:185-200.
- Veldeman M, Höllig A, Clusmann H, Stevanovic A, Rossaint R, Coburn M. Delayed cerebral ischaemia prevention and treatment after aneurysmal subarachnoid haemorrhage: a systematic review. Br J Anaesth. 2016; 117: 17-40.
- Lominadze G, Lessen S, Keene A. Vasospasm risk in surgical ICU patients with grade I subarachnoid hemorrhage. Neurohospitalist. 2016; 6: 20-23.
- Koopman I, van Wijngaarden PB, Rinkel GJE, Vergouwen MDI. Devastating delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Front Neurol. 2022; 13: 2022.
- Hasan D, Lindsay KW, Wijdicks EF, Murray GD, Brouwers PJ, Bakker WH, et al. Effect of fludrocortisone acetate in patients with subarachnoid hemorrhage. Stroke. 1989; 20: 1156-1161.
- Wijdicks EF, Vermeulen M, Hijdra A, van Gijn J. Hyponatremia and cerebral infarction in patients with ruptured intracranial aneurysms: is fluid restriction harmful? Ann Neurol. 1985; 17: 137-140.
- Hasan D, Wijdicks EF, Vermeulen M. Hyponatremia is associated with cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage, AnnNeurol. 1990; 27: 106-108.
- Ziu E, Khan Suheb MZ, Mesfin FB. Subarachnoid hemorrhage. 2017.
- Ogasawara Y, Ito K, Ohkuma H. Atypical presentation of aneurysmal subarachnoid hemorrhage: incidence and clinical importance. Journal of Stroke and Cerebrovascular Diseases. 2016; 25: 1208-1214.
- Holle D, Obermann M. The role of neuroimaging in the diagnosis of headache disorders. Therapeutic advances in neurological disorders. 2013; 6: 369-374.
- Stienen MN, Germans M, Burkhardt JK, Neidert MC, Fung C, Bervini D, et al. Predictors of in-hospital death after aneurysmal subarachnoid hemorrhage: analysis of a nationwide database (Swiss SOS [Swiss Study on Aneurysmal Subarachnoid Hemorrhage]). Stroke. 2018; 49: 333-340.
- Vergouwen MDI, Etminan N, Ilodigwe D, Macdonald RL. Lower incidence of cerebral infarction correlates with improved functional outcome after aneurysmal subarachnoid hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2011; 31: 1545-1553.
- Macdonald RL. Origins of the concept of vasospasm. Stroke. 2016; 47: 11-15.
- Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995; 26: 1086-1092.
- Naredi S, Lambert G, Eden E, Zall S, Runnerstam M, Rydenhag B, et al. Increased sympathetic nervous activity in patients with nontraumatic subarachnoid hemorrhage. Stroke. 2000; 31: 901-906.
- Ogura T, Satoh A, Ooigawa H, Sugiyama T, Takeda R, Fushhara G, et al. Characteristics and prognostic value of acute catecholamine surge in patients with aneurysmal subarachnoid hemorrhage. Neurological Research. 2012; 34: 484-490.
- Cahill J, Calvert J, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2006; 26: 1341-1353.
- Andresen J, Shafi NI, Bryan Jr RM. Endothelial influences on cerebrovascular tone. Journal of applied physiology. 2006; 100: 318-327.
- Otite F, Mink S, Tan CO, Puri A, Zamani AA, Mehregan A, et al. Impaired cerebral autoregulation is associated with vasospasm and delayed cerebral ischemia in subarachnoid hemorrhage. Stroke. 2014; 45: 677-682.
- Yundt KD, Grubb Jr RL, Diringer MN, Powers WJ. Autoregulatory vasodilation of parenchymal vessels is impaired during cerebral vasospasm. Journal of Cerebral Blood Flow & Metabolism. 1998; 18: 419-424.
- Pluta RM, Boock RJ, Afshar JK, Clouse K, Bacic M, Ehrenreich H, et al. Source and cause of endothelin-1 release into cerebrospinal fluid after subarachnoid hemorrhage. Journal of neurosurgery. 1997; 87: 287-293.
- Pluta RM, Afshar JK, Boock RJ, Oldfield EH. Temporal changes in perivascular concentrations of oxyhemoglobin, deoxyhemoglobin, and methemoglobin after subarachnoid hemorrhage. Journal of neurosurgery. 1998; 88: 557-561.
- Ohlstein EH, Storer BL. Oxyhemoglobin stimulation of endothelin production in cultured endothelial cells. Journal of neurosurgery. 1992; 77: 274-278.
- Wickman G, Lan C, Vollrath B. Functional roles of the Rho/Rho kinase pathway and protein kinase C in the regulation of cerebrovascular constriction mediated by hemoglobin: relevance to subarachnoid hemorrhage and vasospasm. Circ Res. 2003; 92: 809-816.
- Sehba FA, Schwartz AY, Chereshnev I, Bederson JB. Acute decrease in cerebral nitric oxide levels after subarachnoid hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2000; 20: 604-611.
- Asano T. Oxyhemoglobin as the principal cause of cerebral vasospasm: a holistic view of its actions. Critical Reviews in Neurosurgery: CR. 1999; 9: 303-318.
- Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. 2009; 40: 1963-1968.
- Keyrouz SG, Diringer MN. Clinical review. Prevention and therapy of vasospasm in subarachnoid hemorrhage. Critical care. 2007; 11: 1-10.
- Kimball MM, Velat GJ, Hoh BL, et al. Critical care guidelines on the endovascular management of cerebral vasospasm. Neurocritical Care. 2011; 15: 336-341.
- Mikeladze KG, Okishev DN, Belousova OB, Konovalov AN, Pilipenko YuV, Ageev IS, et al.: Intra-arterial administration of verapamil for the prevention and treatment of cerebral Angiospasm. Acta Neurochir Suppl. 2020; 127: 179-183.
- Mao G, Gigliotti MJ, Esplin N, Sexton K.: The clinical impact and safety profile of high-dose intra-arterial verapamil treatment for cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Clinical Neurology and Neurosurgery. 2021; 202: 106546.
- Philippon J, Grob R, Dagreou F, Guggiari M, Rivierez M, Viars P. Prevention of vasospasm in subarachnoid haemorrhage. A controlled study with nimodipine. Acta neurochirurgica. 1986; 82: 110-114.
- Liu J, Sun C, Wang Y, Nie G, Dong Q, You J, et al.: Efficacy of nimodipine in the treatment of subarachnoid hemorrhage: a meta- analysis. Arq Neuropsiquiatr. 2022; 80: 663-670.
- Diringer MN, Bleck TP, Hemphill 3rd JC, Menon D, Shutter L, Vespa P, et al.: Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocritical care. 2011; 15: 211- 240.
- Hoh BL, Ko NU, Amin-Hanjani S, H-Y Chou S, Cruz-Flores S, Dangayach NS, et al.: 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/ American Stroke Association. Stroke. 2023; 54: 314-370.