
Research Article
Austin J Clin Neurol 2015;2(1): 1019.
Characterization of Late Recurrence in Long-Term Survivors of Primary Glioblastoma
Gastrell P1, Woodring S2, McSherry F3, Herndon JE II3, Desjardins A4, Friedman H2 and Peters KB4*
1Duke University School of Medicine, USA
2Deparment of Surgery, Duke University Medical Center, USA
3Department of Biostatistics, Duke University Medical Center, USA
4Department of Neurology, Duke University Medical Center, USA
*Corresponding author: Peters KB, Department of Neurology, Preston Robert Tisch Brain Tumor Center at Duke, Duke University Medical Center, P.B: 3624, USA
Received: December 06, 2014; Accepted: February 11, 2015; Published: February 17, 2015
Abstract
Traditionally, overall survival (OS) in primary glioblastoma (GBM) was dismal with 5% at 2 years, but recent advances have improved OS in this population. Long-term survivors (LTS), while rare, can now be identified and evaluated. In a single-center, retrospective analysis, we identified GBM LTS as defined by survival =5 years from diagnosis. To characterize late recurrence in GBM LTS, we extracted a patient subset that experienced disease/treatment free period =2 years. Demographic data was obtained along with characteristics of late recurrence: location, pathology, associated clinical symptoms, and calculation of time to death from late recurrence. 139 primary GBM patients were identified as long-term survivors from January 1, 1998 to August 31, 2011. 42 (30%) had a late recurrence. 59.5% (n=25) were male and average age was 45.6 yrs (range, 23-66yrs). 57.1% (n=24) had new neurological symptoms to indicate recurrence, but the remaining 42.9% were found to have recurrence on serial MRIs. Median OS was 6.8 yrs (95% CI 6.2, 8 years) and median time to late recurrence was 3.6 yrs (95% CI 3.3, 4.6 yrs). Once patients progressed, median time to death from recurrence was 1.3 yrs (95% CI 1, 1.7 yrs) indicating a more aggressive cancer. GBM LTS can develop late recurrences in their disease trajectory even after a protracted disease/treatment free time period. Continued close monitoring with frequent clinical evaluations and MRI imaging is warranted in this population. Establishment of survivorship programs should be considered for GBM LTS to address disease-related and psychosocial issues.
Background
Primary brain tumors represent 1% of all diagnosed cancers, but the survival rates of patients with the most malignant form of these tumors, GBM(WHO grade IV), continues to be poor with less than 3% of patients surviving at five years’ post diagnosis [1,2]. Gliomas in general are the most commonly diagnosed class of primary brain tumor, while GBM remains the most common of the gliomas [1]. Many environmental factors have been proposed to be associated with an increased risk of GBM, with the only unequivocally associated factor being a history of therapeutic X-ray irradiation, especially prophylactic CNS X-ray irradiation in children diagnosed with acute lymphoblastic leukemia [1].
The standard of care for newly diagnosed GBM involves surgical resection followed by temozolomide concurrent with and after radiotherapy. Despite this aggressive approach, median survival has been prolonged only from 12.1 months to 14.6 months relative to radiotherapy alone and disease recurrence and progression occurs with regular frequency [3]. Prognostic factors associated with prolonged survival have been younger age at time of initial diagnosis and high Karnofsky Performance Score at time of diagnosis [2,4-6]. Other factors associated with a good prognosis for survival have included 06-methylguanine DNA methyltransferase (MGMT) promoter methylation status with longer survival being associated with MGMT promoter methylation and isocitrate dehydrogenase 1 mutation [7-9]. Long-term survival in GBM patients has not been studied extensively, and the definition of long-term survival itself has been variably described as either survival greater than three years or survival greater than five year [4,6].
The characteristics of long term survivorship and late GBM recurrence are vital to an understanding of the prognosis and pathophysiology of late recurrence. Furthermore, these characteristics inform physician and patient expectations in the case of long-term survivorship and determine the need for ongoing patient monitoring and psycho-social support. While patient outcomes following GBS diagnosis and treatment have been studied extensively [4,6], a large study characterizing late GBM recurrence in LTS remains absent from the literature. Therefore, we designed a protocol at the Preston Robert Tisch Brain Tumor Center (PRT-BTC) to identify long-term survivorship in primary GBM and describe the factors associated with survivorship and late recurrence.
Methods
Inclusion criteria and data collection
In a retrospective analysis, we identified primary GBM LTS as defined by survival greater than or equal to 5 years from initial GBM diagnosis. Inclusion criteria included a histologically confirmed diagnosis of glioblastoma, treatment at the PRT-BTC between January 1, 1998 and August 31, 2011, ages 18-70 years, and survival of at least 5 years of more following diagnosis of GBM. Clinical characteristics and tumor-related information were collected on all eligible long-term GBM survivors treated at the PRT-BTC, resulting in a population of 139 patients. To characterize late recurrence in GBM LTS, we identified a subgroup of 42 patients who had a diseasefree period for 2 years or greater off therapy before their cancer recurred. Demographic data was obtained from this group along with characteristics of their late recurrence, including tumor location, pathology, KPS at time diagnosis of recurrence, associated clinical symptoms, and calculation of overall survival and time to death from late recurrence.
Statistical analysis
Descriptive statistics were performed on the available demographic characteristics of GBM LTS with recurrence as well as the characteristics of their late recurrence. Kaplan-Meier methods were used to determine and describe median overall survival, median time from the end of treatment to late recurrence, and median time from late recurrence to death. The log-rank test was used to compare patients that were symptomatic at recurrence vs. not symptomatic at recurrence on overall survival and time from late recurrence to death.
Results
Based on the definition of LTS and late recurrence, 42 out of 139 patients (30%) had a late recurrence. Table 1 summarizes the late recurrent patients’ demographic characteristics and the characteristics of their recurrences. Of the 42 patients, 25 (59.5%) were male and 17 (40.5%) were female, and the average age was 45.6 years (range: 23-66 years). Twenty-four patients (57.1%) had neurological symptoms that indicated recurrence, and by August 31st, 2011 32 patients (76.2%) had died.
Table 1: Patient Characteristics.
For all patients with late recurrence, median time from end of treatment for initial primary GBM to late recurrence was 3.6 years (95% CI 3.3, 4.6 years). Figure 1 shows a Kaplan-Meier curve of time from end of treatment to late recurrence. Median overall survival was 6.8 years (95% CI: 6.1, 8.0). Figure 2 shows a Kaplan-Meier curve of overall survival for all recurrent patients following initial diagnosis of GBM. For the long term survivors with late recurrence who eventually died, median time to death after late recurrence was 1.3 years (95% CI: 1.0, 1.7).
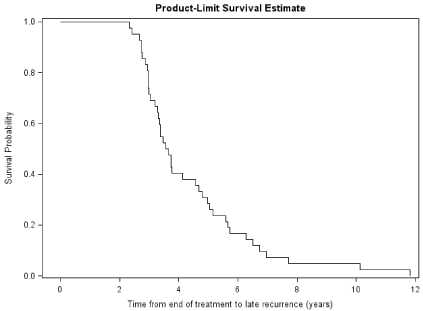
Figure 1: Kaplan-Meier Curve of Time from End of Treatment to Late
Recurrence.
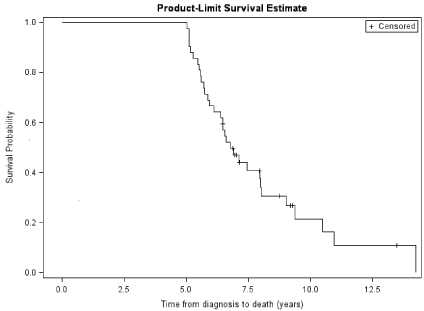
Figure 2: Kaplan-Meier Curve for Overall Survival for All Patients.
Patients who were symptomatic at recurrence had a median overall survival of 6.8 years following initial GBM diagnosis (95% CI: 5.7, 8.0), while patients who were not symptomatic at recurrence had a median overall survival of 7.7 years (95% CI: 5.5, ∞). Median time from late recurrence to death was 1.2 years (95% CI: 0.8, 1.5) for patients who were symptomatic at recurrence and 1.4 years (95% CI: 1.0, 1.8) for patients who were not symptomatic at recurrence. Figure 3 shows the Kaplan-Meier curves comparing overall survival for patients who were symptomatic at recurrence and patients who were not symptomatic at recurrence over time following diagnosis of late recurrence. There was no evidence of a statistical difference in overall survival (log-rank p-value=0.63) or time from late recurrence to death (log-rank p-value=0.86) between the patients who were symptomatic at recurrence and those who were not symptomatic at recurrence.
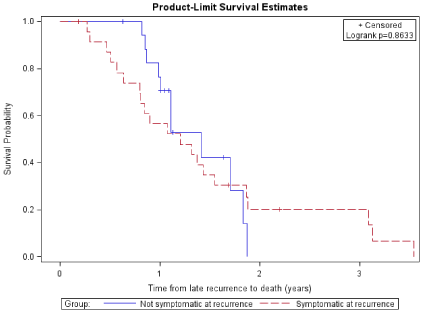
Figure 3: Kaplan-Meier Curve of Time from Late Recurrence to Death for
Patients Symptomatic at Recurrence vs Not Symptomatic at Recurrence
Discussion
Even though this population did experience a longer postdiagnosis survival than most patients with primary GBM, our research has demonstrated recurrent GBM in a significant fraction of LTS. Furthermore, median survival following recurrence of 1.3 years and recurrent patient survival of only 23.8% at the end of our observation period suggests that patients with late recurrent GBS have an overall survival probability and median survival time comparable to primary GBS.
The biological factors associated with late recurrence are vital to an understanding of the prognosis and pathophysiological mechanism of late primary GBM recurrence. Unfortunately, unequivocal research findings describing the factors associated with GBM recurrence remain elusive. Even so, research in this field is ongoing and several studies are now suggesting that alterations in repair proteins in particular mismatch repair, are associated with recurrent tumors and more aggressive phenotypes. Involved repair proteins for mismatch repair include 06-methylguanine-DNA-methyltransferase (MGMT), MSH6, MSH2, and MLH1. In recent study by Felsberg and colleagues, they found the recurrent GBMs express lower levels of MSH2 and MSH6 as detected by immunohistochemistry [10]. Therefore, these mutations are likely to enhance tumorgenicity and lead to radiation and chemotherapeutic resistance. Another compelling possibility is that exposure to alkylator chemotherapy induces an aggressive phenotype. Temozolomide is an alkyating agent that induces a modification in the O6 position of guanine. Two pathways that have been implicated in resistance to temozolomide are the upregulation of MGMT and deficiency of DNA mismatch repair [11]. In in vitro cell lines, a deficiency in mismatch repair prevented the DNA damage induced by temozolomide and hence prevented temozolomide cytotoxicity [12]. In fact, exposure to temozolomide has been shown to be promutagenic, thereby inducing somatic mutations in the DNA in recurrent human malignant gliomas. Furthermore, somatic mutations were also shown to occur at the mismatch repair gene MSH6 [13]. These mutations in MSH6 caused by temozolomide in turn led to deficiency of mismatch repair and resistance to temozolomide. These mutations in the MSH6 were identified in recurrent human malignant gliomas after exposure to temozolomide. This study suggested that induction of mutagenesis of important repair genes by alkylator therapy could in turn lead to resistance to the therapy. Further research showed that MSH6 mutations occurred during therapy with temozolomide and in turn led to progression and/or resistance to temozolomide [14,15].
A deeper understanding of the factors associated with GBM late recurrence can help to inform expectations with regard to disease probability and severity. These factors can also illuminate the pathophysiological mechanisms by which recurrence occurs and guide the treatment of recurrent patients. The standard of care for recurrent GBM is evolving, but may include a second tumor resection or re-irradiation if the patient meets criteria as well as treatment with a number of chemotherapeutic and biological agents including nitrosoureas, temozolomide, and bevacizumab [16]. Positive clinical response to temozolomide therapy may prove unlikely in patients previously treated with temozolomide following initial GBM diagnosis, and strategies to overcome induced tumor resistance have included modified, high-dose metronomic dosing regimens [16]. Unfortunately, combination therapy among multiple therapeutic agents has failed to produce evidence for superior activity, but commonly produces increased drug toxicities [16]. Our understanding of the biology and susceptibilities of normallyoccurring recurrent GBM is clearly evolving, and it remains to be determined if late recurrent GBM represents a physiologically distinct disease entity that warrants and responds to unique treatment measures.
The finding of significant GBS recurrence in LTS and poor survival thereafter raises several issues concerning the management and counseling of long-term GBS survivors. One such issue is the need for continued clinical and radiological surveillance for signs of recurrence five years or more after initial diagnosis of GBM. Currently, there are no clinical trials that define optimal frequency for follow-up after treatment of GBM, while the National Comprehensive Cancer Network recommends that for follow up of primary GBS “a repeat MRI should be obtained four weeks after completion of radiation therapy, then every two to four months for two to three years, and less frequently thereafter.” While tempting for physicians, patients and institutions to cease regular evaluation following a protracted disease free period, our research suggests that even with long term absence of disease following treatment (=2 years), there continues to be a risk for recurrence. Protracted GBS LTS surveillance is thus warranted to maximize prompt diagnosis and treatment of recurrence.
Our study also found no significant difference in terms of median survival or overall survival for patients diagnosed with recurrent GBM who were and who were not symptomatic at time of diagnosis. This raises an interesting question concerning the nature of long-term patient surveillance. Might it be possible that longterm radiographic evaluation may be eventually phased out of a LTS patient surveillance program without impact on patient outcomes following recurrence? Instead, detection of recurrence would rely on patient presentation with new neurological symptoms either after recognition by the patient, patient’s family or other health care providers, or at regular clinical GBS follow-up evaluation. While this is an intriguing possibility, there is currently insufficient research into the characteristics of late recurrence into LTS to determine whether long-term radiography may be withdrawn from a LTS patient surveillance program without affecting patient outcomes.
A final consideration is the psycho-social support a GBS longterm survivor may need given the knowledge that deadly recurrence is possible even after protracted disease free periods. Physicians should be prepared to counsel their patients to remain vigilant for new symptoms for years after diagnosis and patients themselves may benefit from support groups catering to the specific needs and challenges of GBS LTS. Certain behaviors, such as regular exercise, have been associated with an increased OS and increased years of life following initial GBM diagnosis [17]. An ideal GBM LTS support group could address the social and psychological stresses and concerns in the case of GBM LTS as well as educate and encourage patients to adopt behaviors associated with better outcomes.
In conclusion, our research has shown that GBM recurrence remains a significant possibility even after years of disease free status following treatment cessation. Late recurrence is associated with significant mortality, and the survival time following diagnosis of recurrence appears comparable to primary GBM. Unequivocal prognostic factors associated with recurrence probability and severity remains elusive and research into these factors in ongoing. Finally, the high probability of late recurrence in long term survivors indicates a need for patient surveillance programs and psychosocial survivor support catering to the unique needs of GBM LTS.
References
- Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009; 472: 323-342.
- Scott JN, Rewcastle NB, Brasher PM, Fulton D, MacKinnon JA, Hamilton M, et al. Which glioblastoma multiforme patient will become a long-term survivor? A population-based study. Ann Neurol. 1999; 46: 183-188.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352: 987-996.
- Chandler KL, Prados MD, Malec M, Wilson CB. Long-term survival in patients with glioblastoma multiforme. Neurosurgery. 1993; 32: 716-720.
- Salford LG, Brun A, Nirfalk S. Ten-year survival among patients with supratentorial astrocytomas grade III and IV. J Neurosurg. 1988; 69: 506-509.
- Scott JN, Rewcastle NB, Brasher PM, Fulton D, Hagen NA, MacKinnon JA, et al. Long-term glioblastoma multiforme survivors: a population-based study. Can J Neurol Sci. 1998; 25: 197-201.
- Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, et al. Long-term survival with glioblastoma multiforme. Brain. 2007; 130: 2596-2606.
- Sonoda Y, Kumabe T, Watanabe M, Nakazato Y, Inoue T, Kanamori M, et al. Long-term survivors of glioblastoma: clinical features and molecular analysis. Acta Neurochir (Wien). 2009; 151: 1349-1358.
- Zhang GB, Cui XL, Sui DL, Ren XH, Zhang Z, Wang ZC, et al. Differential molecular genetic analysis in glioblastoma multiforme of long- and short-term survivors: a clinical study in Chinese patients. J Neurooncol. 2013; 113: 251-258.
- Felsberg J, Thon N, Eigenbrod S, Hentschel B, Sabel MC, Westphal M, et al. Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int J Cancer. 2011; 129: 659-670.
- Bocangel DB, Finkelstein S, Schold SC, Bhakat KK, Mitra S, Kokkinakis DM, et al. Multifaceted resistance of gliomas to temozolomide. Clin Cancer Res. 2002; 8: 2725-2734.
- Vernole P, Pepponi R, D'Atri S. Role of mismatch repair in the induction of chromosomal aberrations and sister chromatid exchanges in cells treated with different chemotherapeutic agents. Cancer Chemother Pharmacol. 2003; 52: 185-192.
- Hunter C, Smith R, Cahill DP, Stephens P, Stevens C, Teague J, et al. A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res. 2006; 66: 3987-3991.
- Cahill DP, Levine KK, Betensky RA, Codd PJ, Romany CA, Reavie LB, et al. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res. 2007; 13: 2038-2045.
- Yip S, Miao J, Cahill DP, Iafrate AJ, Aldape K, Nutt CL, et al. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res. 2009; 15: 4622-4629.
- Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro Oncol. 2013; 15: 4-27.
- Ruden E, Reardon DA, Coan AD, Herndon JE 2nd, Hornsby WE, West M, et al. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol. 2011; 29: 2918-2923.