
Review Article
Austin J Clin Neurol 2015;2(4): 1037.
Diabetic Hemichorea-hemiballism
Ohara S*
Department of Neurology, Matsumoto Medical Center, Japan
*Corresponding author: Shinji Ohara, Department of Neurology, Matsumoto Medical Center, Chushin- Matsumoto Hospital, 811 Kotobuki, Matsumoto 399-0021, Japan
Received: February 05, 2015; Accepted: March 10, 2015; Published: April 09, 2015
Abstract
Hemichorea/hemiballism (HC/HB) associated with nonketotic hyperglycemia (NKH) and hyperintense putamen on T1 weighted MRI has been recognized as a clinical entity, but the substrate responsible for the MRI signal changes and the pathogenesis of HC/HB remain unsettled. This syndrome is relatively rare, but its recognition is important because the correction of hyperglycemia usually leads to prompt clinical and radiological improvement, and occasionally to the detection of untreated diabetes mellitus. In this review, the clinical, radiological, and pathological features of this syndrome are extensively described with reference to possible underlying mechanisms.
Keywords: Nonketotic hyperglycemia; Hemichorea; Hemiballism; Diabetes mellitus; Magnetic resonance imaging
Abbreviations
HC/HB: Hemichorea/hemiballism; DM: Diabetes Mellitus; NKH: Nonketotic Hyperglycemia; NPY: Neuropeptide Y
Introduction
Hemichorea/hemiballism (HC/HB) is a continuous, arrhythmic hyperkinetic movement disorder occurring in a distal or proximal part of body. Chorea is characterized by jerky, forcible, often twisting movements of an arm or the trunk or facial grimacing, that can be incorporated into voluntary movements, and ballism is characterized by proximal dominant, often violent limb-throwing-like movement of the entire limb [1]. Chorea and ballism often coexist and blend with each other in a single patient, so that the difference may be due simply to the difference in the amount of movement and not in quality. The anatomical lesions responsible for HC/HB are generally found in the contralateral subthalamic nucleus and/or the basal ganglia. The varying etiologies include cerebrovascular, inflammatory, infectious, metabolic/toxic, and neurodegenerative etiologies, and those related to neoplasms or that are paraneoplastic. Autoimmune and infectious etiologies are more common among young individuals compared to the elderly, whereas cerebrovascular accidents are the most common etiology among the elderly, often with a background of concomitant hypertension and/or diabetes mellitus (DM).
Non-ketotic hyperglycemia (NKH) has been known to be associated with chorea and/or ballism [2-4]. However, NKH has gained increasing attention since Yahikozawa et al. reported a new syndrome in 1994 that they named “hemiballism with striatal hyperintensity on T1-weighted MRI in diabetic patients” [5]. At present, this syndrome is regarded as the second most common cause of HC/HB, following cerebrovascular accidents.
The clinical importance of this syndrome lies in the fact that it can be the first manifestation of DM, and that, in most cases; it can be improved simply by the correction of hyperglycemia and an intravenous administration of fluids. Because of the relatively benign course of the symptoms of this syndrome, the underlying pathogenesis of this condition has been unclear.
Clinical features
The ‘hemiballism with striatal hyperintensity on T1-weighted MRI in diabetic patients’ syndrome has been reported most often in patients from Japan and Asian countries and was found more commonly in elderly females with diabetes. Among 53 patients with this syndrome, Oh et al. reported that their mean age was 71 years, the female to male ratio was 1.8:1, and the mean hemoglobin A1C (HbA1C) was 14% [6].
The typical clinical course is as follows; an elderly patient with history of DM presents to an emergency room with an acute or subacute onset of HC/HB. Imaging studies of the brain to rule in/ out cerebrovascular stroke reveals high intensities on computed tomography (CT) and high signals in T1 MRI in the striatum contralateral to the side of the involuntary movement. Laboratory studies reveal NKH. After the correction of blood sugar, the involuntary movement as well as abnormal imaging resolves gradually [5-7].
The HC/HB is usually unilateral, but can involve both sides when striatal lesions are bilateral [6-8]. Once resolved following the correction of blood sugar, HC/HB may recur with resurgent hyperglycemia [9]. Correction of the hyperglycemia does not necessarily lead to abolishment of the involuntary movement and may lag for a few years [10,11].
Weakness or paresthesias may precede the appearance of HC/ HB on the same side of the body [12,13]. The level of consciousness usually maintained, though in rare cases, alteration in consciousness, generalized seizures, or changes in the personalities may precede the appearance of HC/HB [10]. The latter may be associated with a disturbance of striatal-frontal projections, which may represent a non-motor feature of this syndrome [14].
Typically, T1 weighted MRI reveals high signals in the striatum of patients with this syndrome. However, cases presenting with hyperglycemia and acute onset chorea but with normal MRI imaging have been reported [11,14,15]. Cases presenting hyperglycemia and typical T1 MRI imaging abnormalities without involuntary movement have also been reported [16,17]. Imaging abnormalities on MRI or CT may precede the appearance of HB/HC [18,19]. It is possible that these variances reflect differences in the stage and/or degree of a common underlying pathology.
It is important to note that the appearance of hemiballism/ hemichorea may be the first manifestation of diabetes [20,21].
The previous reports of this syndrome have been mostly of elderly patients with poorly controlled type II diabetes. However, cases of previously healthy adolescents presenting with hemichorea as an initial manifestation of type I diabetes have been reported with or without MRI abnormalities, suggesting that this syndrome could also occur in childhood [15, 22].
Imaging studies
The characteristic MRI feature of this syndrome is high and relatively uniform signal intensity of the putamen conforming to the anatomical contour on T1 weighted images. Generally, this is not accompanied by mass effect or contrast enhancement. In majority of cases, T2 weighed MRI revealed low signals in the striatum. CT scans reveals high-intensity of the putamen. But it is less conspicuous than that on T1-MRI. Less often, the globus pallidus and caudate nucleus are involved, though internal capsules are generally spared from signal abnormalities. Patients presenting with hemichorea may show bilateral abnormal signals on T1 MRI.
Typical chronological changes of this syndrome on CT, MRI and single-photon emission computed tomography (SPECT) are as follows [6,23]. In the acute stage, there are no apparent edematous changes of the striatum itself and no surrounding edema. Following recovery, abnormal signals improve first with CT, followed by MRI. There is no conversion of signals from high to low or vice versa during the follow-ups. Unless the recovery is protracted, atrophic changes are not generally apparent after the disappearance of highintensity signals on T1-weighted MRI. SPECT studies may reveal hyperperfusion of the basal ganglia on the contralateral side in the acute stage and hypo-perfusion in the late stage.
With regard to T2-weighted gradient-echo MRI findings, in which hemorrhagic lesions can be depicted as low signals, there have been conflicting reports. Some groups reported that there were no abnormalities [24,25] and others described low signals [26-28]. The findings on diffusion MRI have been variable; some researchers reported increased signals in the putamen [9,12,16], whereas others did not [12,27].
It is noteworthy that hyperglycemic hemiballism may reveal lesions not only in the striatum but also the subthalamic nucleus at contralateral side of the involuntary movement. Kim et al. reported that a patient who presented with hemiballism and nonketotic hyperglycemia showed a localized subthalamic lesion on MRI imaging [29]. In this patient, only the subthalamic nucleus appeared high on T1 and low on T2. On the other hand, Maeda et al. reported an autopsied case of hyperglycemic hemiballism with negative MRI findings. The autopsy revealed a localized lesion in the contralateral subthalamic nucleus.
Pathology
There have been only a few autopsy [30-32] and biopsy [12,18,33] reports of patients with hyperglycemic HC/HB associated with characteristic T1 MRI findings in the putamen. In the first autopsy report, the characteristic T1 MRI hyperintensity in the putamen was recognized 18 days prior to the patient’s death [30]. The autopsy revealed an increased number of reactive astrocytes and the presence of scattered small foci of fresh tissue necrosis. This was associated with enhanced neuropeptide Y (NPY) immunoreactivity of the interneurons. The contralateral putamen revealed only old lacunar infarctions which had been apparent on MRI images. No hemorrhagic changes, either fresh or old, could be identified histologically.
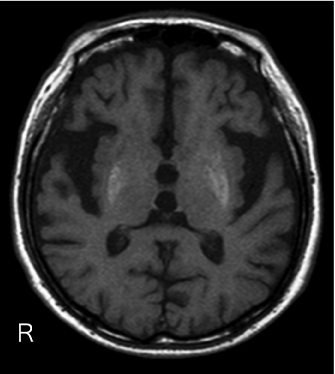
Figure 1: Axial T1-weighted magnetic resonance imaging (MRI) of an 89 yearold
man with history of poorly controlled diabetes presented with nonketotic
hyperglycemia and right sided hemiballism and orofacial dyskinesia, showing
diffuse high intensity of the bilateral putamen and globus pallidus which was
more intense on the left than right side. R: Right.
Another of the three autopsy cases was a patient with diabetic hemiballisms refractory to the correction of blood sugar who died 36 days after the onset. The striatum, where T1-weighted MRI revealed characteristic hyperintensity, also showed acute ischemic changes with neuronal necrosis associated with the proliferation of reactive astrocytes and microhemorrhages [31]. The third autopsy case was that of a patient who presented with bilateral chorea and T1MRI showing irregular/-not homogenous abnormalities in the bilateral putamina. The autopsy revealed multiple lacunar infarcts and the presence of phagocytes associated with surrounding reactive astrocytosis and pericapillary hemosiderin deposition [32].
From these autopsy reports, it seems apparent that the pathologic changes responsible for the characteristic T1-weighted MRI signals in this syndrome is the diffuse proliferation of reactive astrocytes associated with ischemic necrosis with or without the presence of microhemorrhages. With a biopsy fragment of the striatum that had showed high intensity on T1-weighted MRI, proliferation of hypertrophic astrocytes have been reported [18,33]. Abe et al. reported vascular changes including capillary proliferation and hyaline degeneration of the arteriolar walls associated with ischemic necrosis in a striatal biopsy. They suggested that the histological changes are analogous to those of proliferative retinopathy [12].
Maeda et al. reported the autopsy findings of a 59-year-old patient with diabetes who had presented with an upper limb involuntary movement which progressed to hemiballism within 3 days [34]. The brain MRI on admission was unremarkable and the correction of the patient’s blood sugar resulted in the rapid improvement of the involuntary movement. The patient died suddenly on day 57 from possible fatal arrhythmias, based on severe autonomic neuropathy. The autopsy revealed only involvement of the contralateral subthalamic nucleus as revealed by the presence of activated microglia. It is of great interest that the lesion was not associated with reactive astrocytosis, which may indicate that microglial activation may be the earliest pathological feature of this syndrome.
In neuropathological examinations, immunohistochemistry for reactive astrocytes or microglia is widely used as sensitive markers of neural damages. Both astrocytes and microglias have long been regarded as supportive components of the nervous tissue which could be activated and transform into the reactive state. It has become increasingly evident that the reaction of astrocytes and microglia may not be nonspecific, but rather may be neuroprotective or neurodestructive through the production and release of neurotrophic factors and cytokines depending on the circumstances [35,36]. The roles played by activated astrocytes and/or microglia in this syndrome remain to be identified.
Etiology and pathogenesis
Based on the anatomical model of the basal ganglia-thalamuscerebral cortices circuit, disinhibition of the neural activities of the subthalamic nucleus and subsequent activation of the motor cortex occur by way of thalamo-cerebral projection pathways [37]. In the two reported cases of diabetic hemiballism, only the subthalamus was involved as revealed radiologically [29] or pathologically [30]. In the vast majority of the reported cases in which abnormal high intensity was found in the putamina on T1-weighted MRI, the occurrence of abnormal excitation of thalamo-cortical projections has been implicated based on SPECT showing that the cerebral blood flow was increased in the thalamus and decreased in the putamen contralateral to the side of involuntary movement [38]. This implication has been underscored neurophysiologically. Goto et al. studied pallidal neuronal activity in diabetic HC/HB by using microelectrode, and they found decreased firing rate in the internal globus pallidum (GPi), which suggested increased activity in the direct striatum-GPi inhibitory pathways [39].
Two major hypotheses have been proposed to explain the pathogenesis of the striatal lesions; metabolic versus ischemic pathogenesis.
NKH is known to suppress the secretion of insulin, which results in disturbed intracellular transport of glucose. In the metabolic hypothesis, the shortage of available glucose leads to a depletion of GABA, which is recruited as a substrate of citrate cycle (GABA shunt). This leads to impaired function of GABAergic inhibitory neurotransmission in the striatum and the disinhibition of basal ganglia-thalamo-cortical circuit, resulting in abnormal involuntary movement. The correction of hyperglycemia leads to the normalization of GABA, resulting in the disappearance of abnormal movement. It has been shown, in rodents, that GABA level and the contribution of GABA to neurotransmitter activities are significantly higher in the striatum and thalamus than in other brain regions including the cerebral cortices, suggesting that the selective activation of inhibitory neurons would result in a higher functional signal in these areas [40]. However, this metabolic hypothesis alone cannot explain the unilateral nature of the striatal involvement described in the vast majority of the reported cases of this syndrome, and it cannot explain why resistant cases exist in which involuntary movement continues despite the normalization of hyperglycemia.
The second hypothesis is based on regional ischemia. The populations who are susceptible to this syndrome are elderly patients with poorly controlled DM, often afflicted with diabetic vasculopathy. It has been established that ischemic tissue injuries tend to worsen or become hemorrhagic due presumably to disruption of the blood brain barrier [41, 42]. Under the dehydration of the hyperviscosity state induced by NKH [43], a thrombotic obstruction of vessels or transient ischemia of a hemodynamic nature may be liable to occur, although it is to be noted that obstruction of the microvascular lumens in the affected striatum has seldom been observed histologically [12,18,26,30-33].
In the experimentally induced rat model of short-lasting cerebral ischemia, the putamen showed MRI features closely resembling those of this syndrome. The histology of the putamen revealed incomplete ischemic lesions manifested by selective neuronal loss and a proliferation of astrocytes [44,45]. Fujioka et al. further demonstrated that such ischemia results in the deposition of manganese ions in reactive astrocytes and cause a paramagnetic effect [46], which is consistent with the view that gemistocytes are sufficient to explain the shortening of the T1 relaxation time [18]. However, the ischemic hypothesis cannot explain the fact that the correction of hyperglycemia alone commonly resulted not only in the disappearance of HC/HB but also the reversal of abnormal MRI findings. In addition, the fact that recurrence of the involuntary movement can occur with worsening of the hyperglycemia or superimposed infections cannot be explained by the ischemic hypothesis alone.
The metabolic and ischemic hypotheses are not mutually exclusive; they may be closely related pathogenetically to each other. A case of hyperglycemic hemichorea was reported to be associated with vascular anomalies in the contralateral putamen [47]. A patient with hyperglycemic HC/HB with typical radiological striatal features was found to have unilateral carotid occlusive disease [48]. Thus, metabolic disturbances may unmask a previously unrecognized asymptomatic vasculopathy. It is possible that in patients with poorly controlled diabetes, vasculopathy and its regional chronic ischemia may make one side of the striatum more vulnerable to systemic metabolic stress than the other side, resulting in a unilateral appearance of HC/HB.
Differential diagnosis
Blood glucose determination is essential when a patient present with acute onset of HC/HB. If hyperglycemia is present and MRI of the brain reveals T1 high intensity in the putamen, the diagnosis of this syndrome is highly likely. If attending physicians are unaware of this syndrome, their patients may be referred to neurosurgery service for suspicion of a putaminal hemorrhage or brain tumor, which may lead to an unnecessary brain biopsy or surgery.
When a patient’s involuntary movement is not resolved after the correction hyperglycemia, or when MRI findings of the putamen are not characteristic of this syndrome, further laboratory investigation is necessary. The differential diagnoses can be broad including cerebrovascular disorders, metabolic disorders other than diabetes, infectious or inflammatory disorders, autoimmune disorders,paraneoplastic striatal inflammation and more. Possible non-organic etiologies include conversion reaction (hysteria) and focal motor seizures. The latter could be caused by hyperglycemia alone, so that in doubtful cases an electroencephalogram (EEG) is indicated.
In contrast to HC/HB caused by cerebrovascular stroke, which is usually monophasic, symptom relapses are often observed in diabetic hemiballism/chorea. In general, relapse and/or fluctuation of symptoms are features of involuntary movements due to metabolic disorders, known examples being chorea due to hyperthyroidism or to an electrolyte disturbance such as hypernatremia. These are generally bilateral in occurrence and not associated with imaging abnormalities of the basal ganglia. Of note, in cases of chorea associated with uremia secondary to diabetic nephropathy, abnormal signals could be recognized in the basal ganglia with MRI. However, unlike cases of diabetic HC/HB, they reveal T1 low and T2 high intensities [49].
It should be noted that, in juvenile-onset chorea, hyperglycemic HC/HB could be a manifestation of mitochondrial cytopathy. There have been two case reports of juvenile patients who presented with hyperglycemic chorea and high intensity lesions in the basal ganglia on T1-weighted MRI, subsequently diagnosed as mitochondrial encephalopathy, lactic acidosis and strole-like episodes (MELAS) with A3243G mutation [50,51].
Neurodegenerative diseases are often accompanied by the appearance of chorea, such as Huntington’s disease or neuroacantocytosis. However, HC/HB and these disorders can be easily differentiated based on other clinical features and imaging studies.
Therapy and prognosis
Following the correction of patient’s hyperglycemia, the intensity of HC/HB usually but not always gradually resolves and disappears. The resolution of abnormal intensities on T1 MRI tends to lag behind the improvement of the involuntary movement [6,7]. In some patients, the involuntary movement persists regardless of the improvement of the abnormal intensities on MRI images [10,11,23]. Wu et al. reported a patient with persistent HC/HB lasting for 6 months associated with the appearance of periodic synchronized discharges (PLEDs) on EEG, suggesting that the appearance of PLEDs may indicate an irreversible outcome in this syndrome [52]. In addition, because this syndrome tends to occur in elderly patients with poorly controlled DM, complications such as pneumonia, sepsis and cerebral infarction are not uncommon, and these may seriously alter the outcome of therapy and prognosis.
When the involuntary movement would not resolve after the correction of blood sugar, pharmacotherapy for chorea or ballism may be indicated [1]. The first-line agents are dopamine-blocking agent such as haloperidol. When this is not effective, risperidone, tiapride, sulpiride, diazepam could be tried. For medication-resistant HC/HB, functional neurosurgery may be indicated. Deep brain stimulation of the thalamus [53] and pallidotomy [38], have been successfully applied to patients with resistant HC/HB. More recently, repetitive transcranial magnetic stimulation was effective in a patient with persistent (> yr) hemichorea associated with atrophic changes in the contralateral striatum [54].
Conclusion
Since the first description of diabetic HC/HB associated with T1 high intensity of the putamen, this syndrome has been recognized as a distinct clinical entity. This syndrome tends to occur in elderly patients with poorly controlled DM, but it can also be a first manifestation of unrecognized DM. In most instances, the correction of hyperglycemia can bring about significant improvements of both the clinical symptoms and the imaging abnormalities. The recognition of this treatable clinical entity is thus important. However, atypical or resistant cases, and cases with only subthalamic involvement have been reported, suggesting that the spectrum of this syndrome may be more varied than originally described. Metabolic and circulatory disturbances may both be contributory, but the exact pathogenesis of this syndrome remains to be established.
Acknowledgement
I thank Dr. Tomoko Ohtsuki and Dr. Masao Ushiyama, Department of Internal Medicine and Neurology, Kenwa-kai hospital, Iida, Japan for their kindly providing typical MRI images of a patient with this syndrome for this review.
References
- Postuma RB, Lang AE. Hemiballism: revisiting a classic disorder. Lancet Neurol. 2003; 2: 661-668.
- Bedwell SF. Some observations on hemiballismus. Neurology. 1960; 10: 619-622.
- Schwarz GA, BARROWS LJ. Hemiballism without involvement of Luys' body. Arch Neurol. 1960; 2: 420-434.
- Rector WG Jr, Herlong HF, Moses H 3rd. Nonketotic hyperglycemia appearing as choreoathetosis or ballism. Arch Intern Med. 1982; 142: 154-155.
- Yahikozawa H, Hanyu N, Yamamoto K, Hashimoto T, Shimozono K, Nakagawa S, et al. Hemiballism with striatal hyperintensity on T1-weighted MRI in diabetic patients: a unique syndrome. J Neurol Sci. 1994; 124: 208-214.
- Oh SH, Lee KY, Im JH, Lee MS. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci. 2002; 200: 57-62.
- Lin JJ, Lin GY, Shih C, Shen WC. Presentation of striatal hyperintensity on T1-weighted MRI in patients with hemiballism-hemichorea caused by non-ketotic hyperglycemia: report of seven new cases and a review of literature. J Neurol. 2001; 248: 750-755.
- Sung YH, Park KH, Lee YB, Park HM, Shin DJ. Chorea in the both lower limbs associated with nonketotic hyperglycemia. J Mov Disord. 2009; 2: 98-100.
- Wintermark M, Fischbein NJ, Mukherjee P, Yuh EL, Dilon WP. Unilateral putaminal CT, MR, and diffusion abnormalities secondary to nonketotic hyperglycemia in the setting of acute neurologic symptoms mimicking stroke. Am J Neuroradiol. 2004; 25: 975-976.
- Chung SJ, Lee JH, Lee SA, No YJ, Im JH, Lee MC. Co-occurrence of seizure and chorea in a patient with nonketotic hyperglycemia. Eur Neurol. 2005; 54: 230-232.
- Ahlskog JE, Nishino H, Evidente VG, Tulloch JW, Forbes GS, Caviness JN, et al. Persistent chorea triggered by hyperglycemic crisis in diabetics. Mov Disord. 2001; 16: 890-898.
- Abe Y, Yamamoto T, Soeda T, Kumagai T, Tanno Y, Kubo J, et al. Diabetic striatal disease: clinical presentation, neuroimaging, and pathology. Intern Med. 2009; 48: 1135-1141.
- Taboada GF, Lima GA, Castro JE, Liberato B. Dyskinesia associated with hyperglycemia and basal ganglia hyperintensity: report of a rare diabetic complication. Metab Brain Dis. 2013; 28: 107-110.
- Kranick SM, Price RS, Prasad S, Hurtig HI. Clinical reasoning: a 52-year-old woman with subacute hemichorea. Neurology. 2008; 71: e59-62.
- Mihai CM, Catrinoiu D, Stoicescu RM. Atypical onset of diabetes in a teenage girl: a case report. Cases J. 2008; 1: 425.
- Hsu JL, Wang HC, Hsu WC. Hyperglycemia-induced unilateral basal ganglion lesions with and without hemichorea. A PET study. J Neurol. 2004; 251: 1486-1490.
- Sorimachi T, Fujii Y, Tsuchiya N, Saito M. Striatal hyperintensity on T1-weighted magnetic resonance images and high-density signal on CT scans obtained in patients with hyperglycemia and no involuntary movement. Report of two cases. J Neurosurg. 2004; 101: 343-346.
- Shan DE, Ho DM, Chang C, Pan HC, Teng MM. Hemichorea-hemiballism: an explanation for MR signal changes. AJNR Am J Neuroradiol. 1998; 19: 863-870.
- Nakajima N, Ueda M, Nagayama H, Katayama Y. Putaminal changes before the onset of clinical symptoms in diabetic hemichorea-hemiballism. Intern Med. 2014; 53: 489-491.
- Felicio AC, Chang CV, Godeiro-Junior C, Okoshi MP, Ferraz HB. Hemichorea-hemiballism as the first presentation of type 2 diabetes mellitus. Arq Neuropsiquiatr. 2008; 66: 249-250.
- Bekiesinska-Figatowska M, Romaniuk-Doroszewska A, Banaszek M, Kuczynska-Zardzewialy A. Lesions in basal ganglia in a patient with involuntary movements as a first sign of diabetes - case report and review of the literature. Pol J Radiol. 2010; 75: 61-64.
- Aquino JH, Spitz M, Pereira JS. Hemichorea-Hemiballismus as the First Sign of Type 1b Diabetes During Adolescence and Its Recurrence in the Setting of Infection. J Child Neurol. 2014;.
- Hashimoto T, Hanyu N, Yahikozawa H, Yanagisawa N. Persistent hemiballism with striatal hyperintensity on T1-weighted MRI in a diabetic patient: a 6-year follow-up study. J Neurol Sci. 1999; 165: 178-181.
- Omori H, Hirashima K, Ishihara D, Maeda Y, Hirano T, Uyama E, et al. Two cases of hemiballism-hemichorea with T1-weighed MR image hyperintensities. Intern Med. 2005; 44: 1280-1285.
- Chu K, Kang DW, Kim DE, Park SH, Roh JK. Diffusion-weighed and gradient echo magnetic resonance findings of hemichorea-hemiballismus associated with diabetic hyperglycemia: a hyperviscosity syndrome? Arch Neurol. 2002; 59: 448- 452.
- Lai PH, Tien RD, Chang MH, Teng MM, Yang CF, Pan HB, et al. Chorea-ballismus with nonketotic hyperglycemia in primary diabetes mellitus. AJNR Am J Neuroradiol. 1996; 17: 1057-1064.
- Suto Y, Mori M, Kagimoto H, Saito J. [A case of hemichorea with hyperglycemia presenting with low signal intensity in the striatum on T2*-weighted gradient-echo magnetic resonance imaging]. Rinsho Shinkeigaku. 2004; 44: 86-90.
- Ishibashi M, Kikuchi A, Takeda A, Onodera J. A case of diabetic hemichorea with petechial hemorrhages in the striatum - investigation by T2 weighted MRI and MRS. Jpn J Stroke. 2006; 28: 301-305.
- Kim HJ, Moon WJ, Oh J, Lee IK, Kim HY, Han SH. Subthalamic lesion on MR imaging in a patient with nonketotic hyperglycemia-induced hemiballism. AJNR Am J Neuroradiol. 2008; 29: 526-527.
- Ohara S, Nakagawa S, Tabata K, Hashimoto T. Hemiballism with hyperglycemia and striatal T1-MRI hyperintensity: an autopsy report. Mov Disord. 2001; 16: 521-525.
- Nath J, Jambhekar K, Rao C, Armitano E. Radiological and pathological changes in hemiballism-hemichorea with striatal hyperintensity. J Magn Reson Imaging. 2006; 23: 564-568.
- Mestre T, Ferreira JJ, Pimental J. Putaminal petechial haemorrhages as the cause of non-ketotic hyperglycaemic chorea: a neuropathological case correlated with MRI findings. J Neurol Neurosurg Psychiat. 2007; 78: 549-550.
- Nakamura K, Akamine T, Makihara S, Asami N, Yamakawa Y. Hemiballism presenting with high intensity at lentiform nuclei on short spin echo of serial MRI. A case report. Rinsho Shinkeigaku (Clin Neurol). 1992; 36: 203-206.
- Maeda K, Katayama Y, Sugimoto T, Somura M, Kajino Y, Ogasawara K, et al. Activated microglia in the subthalamic nucleus in hyperglycaemic hemiballism: a case report. J Neurol Neurosurg Psychiatry. 2010; 81: 1175-1177.
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997; 20: 570-577.
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010; 58: 253-263.
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990; 13: 281-285.
- Kim JS, Lee KS, Lee KH, Kim YI, Kim BS, Chung YA, et al. Evidence of thalamic disinhibition in patients with hemichorea: semiquantitative analysis using SPECT. J Neurol Neurosurg Psychiatry. 2002; 72: 329-333.
- Goto T, Hashimoto T, Hirayama S, Kitazawa K. Pallidal neuronal activity in diabetic hemichorea-hemiballism. Mov Disord. 2010; 25: 1295-1297.
- Tiwari V, Ambadipudi S, Patel AB. Glutamatergic and GABAergic TCA cycle and neurotransmitter cycling fluxes in different regions of mouse brain. J Cereb Blood Flow Metab. 2013; 33: 1523-1531.
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001; 32: 2426-2432.
- Kagansky N, Levy S, Knobler H. The role of hyperglycemia in acute stroke. Arch Neurol. 2001; 58: 1209-1212.
- Pisani A, Diomedi M, Rum A, Cianciulli P, Floris R, Orlacchio A, et al. Acanthocytosis as a predisposing factor for non-ketotic hyperglycaemia induced chorea-ballism. J Neurol Neurosurg Psychiatry. 2005; 76: 1717-1719.
- Fujioka M, Taoka T, Matsuo Y, Hiramatsu K, Sakaki T. Novel brain ischemic change on MRI. Delayed ischemic hyperintensity on T1-weighed images and selective neuronal death in the caudoputamen of rats after brief focal ischemia. Stroke. 1999; 30: 1043-1046.
- Aoe H, Takeda Y, Kawahara H, Tanaka A, Morita K. Clinical significance of T1-weighted MR images following transient cerebral ischemia. J Neurol Sci. 2006; 241: 19-24.
- Fujioka M, Taoka T, Matsuo Y, Mishima K, Ogoshi K, Kondo Y, et al. Magnetic resonance imaging shows delayed ischemic striatal neurodegeneration. Ann Neurol. 2003; 54: 732-747.
- Kimura A, Mitake S. [Repeated hyperglycemic clin Neurol in a patient with venous angioma in the putamen]. Rinsho Shinkeigaku. 2001; 41: 113-116.
- Ahmad A, Paliwal P, Wakerley BR, Teoh HL, Sharma VK. Vascular contribution to hyperglycaemia-induced hemichorea. Diab Vasc Dis Res. 2013; 10: 378-379.
- Lee EJ, Park JH, Ihn Yk, Kim YJ, Lee SK, Park CS. Acute bilateral basal ganglia lesions in diabetic uraemia: diffusion-weighted MRI. Neuroradiology. 2007; 49: 1009-1013.
- Nakagaki H, Furuya J, Santa Y, Nagano S, Araki E, Yamada T. [A case of MELAS presenting juvenile-onset hyperglycemic chorea-ballism]. Rinsho Shinkeigaku. 2005; 45: 502-505.
- Kang JH, Kang SY, Choi JC, Lee SS, Kim JS. Chorea triggered by hyperglycemia in a maternally inherited diabetes and deafness (MIDD) patient with the A3243G mutation of mitochondrial DNA and basal ganglia calcification. J Neurol. 2005; 252: 103-105.
- Wu MN, Ruge D, Tsai CL, Hsu CY, Lai CL, Liou LM. Periodic Lateralized Epileptiform Discharges Associated With Irreversible Hyperglycemic Hemichorea-Hemiballism. Clin EEG Neurosci. 2014.
- Nakano N, Uchiyama T, Okuda T, Kitano M, Taneda M. Successful long-term deep brain stimulation for hemichorea-hemiballism in a patient with diabetes. Case report. J Neurosurg. 2005; 102: 1137-1141.
- Kaseda Y, Yamawaki T, Ikeda J, Hayata M, Dohi E, Ohshita T, et al. Amelioration of persistent, non-ketotic hyperglycemia-induced hemichorea by repetitive transcranial magnetic stimulation. Case Rep Neurol. 2013; 5: 68-73.