
Research Article
Austin J Clin Neurol 2015;2(6): 1052.
Efficacy of Botulinum Toxin-A Treatment in Chronic Migraine – Kuwait Experience
Al-Hashel JY1,2*, Nagarajan V¹ and Ahmed SF1,3
¹Department of Neurology, Ibn Sina Hospital, Kuwait
²Neurologist, Kuwait University, Kuwait
³Department of Neurology, Minia University, Egypt
*Corresponding author: Jasem Yousef Al-Hashel,Department of Neurology, Ibn Sina Hospital, P.O. Box 25427, Safat 13115, Kuwait
Received: April 13, 2015; Accepted: May 08, 2015; Published: May 19, 2015
Abstract
Background: Botulinum toxin-A (BoNT-A) is approved for prophylactic treatment of chronic migraine (CM). We aimed to assess the efficacy and safety of BoNT-A in the treatment of CM.
Methods: This open-label prospective study included 40 CM patients. Each patient received 100 units of BoNT-A following fixed site fixed dose protocol. Patient’s headache was assessed by their headache dairy and recording Headache Impact test (HIT-6) at baseline and 4th, 8th and 12th weeks following BoNT-A injection. Adverse events (AEs) were monitored to assess the safety of BoNT-A. For willing patients, BoNT-A injection was given and they were assessed at 3 months interval.
Results: After BoNT-A treatment, there were reduction in all parameters (headache frequency and severity, analgesic consumption and HIT-6 score) by 40% at 4th week, 45% at 8th weeks and 42% at 12th week post treatment. At 4th week, 62.5% of patients achieved good response while, 37.5% indicated no alteration in their headache frequency and severity. At 8th week and 12th week post treatment 30%, 25% respectively were found to have no response to treatment. Five patients (12.5%) experienced mild and short lasting AEs. There was 70% improvement of variables after repeated injections at 3 months interval.
Conclusions: BoNT-A is effective and well tolerated therapy in the prophylaxis of CM.
Keywords: Chronic Migraine; Migraine; Botulinum Toxin-A; Botox; Kuwait; Middle East
Introduction
Chronic migraine (CM) is a disabling, underdiagnosed, and undertreated neurological disorder [1]. Currently, CM is defined as Headache occurring on 15 or more days per month for more than 3 months, which has the features of migraine headache on at least 8 days per month meeting criteria for migraine without aura or demonstrating response to migraine-specific treatment [2]. It is estimated that about 2% of general population meets the criteria for chronic migraine with or without analgesic overuse [3]. Chronic migraine is important not only on account of its great frequency, but also because it significantly reduces the quality of life of the patients [4].
Chronic migraine is very difficult to treat even by the experts. Currently there are no agent approved for CM prophylaxis and whatever available symptomatic medications have significant adverse effects (AEs) with limited benefit. So there is always a demand for useful alternative preventive therapies with limited AEs among CM sufferers [5]. Despite advances in headache therapies, there remains a group of sufferers with refractory (intractable, treatment-resistant) headache who fail to respond to or cannot tolerate current evidencebased treatments [6]. Refractory chronic migraine is often associated with disability and a low quality of life (QOL) [7].
Since 2010, based on the two PREEMPT-studies (Phase III Research Evaluating Migraine
Prophylaxis Therapy), Botulinum toxin A (BoNT-A) is registered for the indication chronic migraine in the USA and since 2011 in Great Britain and the European Community [8].
BoNT-A mediates its postulated mechanism of action in migraine by inhibiting the release of neurotransmitters in the cholinergic (motor and autonomic) and nociceptive (sensory) nerve endings such as glutamate, substance P, calcitonin gene-related peptide and acetylcholine so, it temporarily relieve the muscle spasm, regulate the exocrine secretions and reduce the neurogenic inflammation responsible for pain [9]. The advantages of a treatment with BoNT-A are the good tolerability, the lack of side-effects and the therapeutic effect over three to six months. The success rate varies between 30% and 50% [8]. Thus, BoNT-A may be an ideal prophylactic agent for use in patients with CM.
The purpose of our prospective, open-label study was to assess the efficacy and safety of BoNT-A in the treatment of CM patients in Kuwait.
Material and Methods
Study design
This is open-label prospective study that was conducted in Ibn Sina hospital, Kuwait which is a tertiary neurology care centre that having regular Headache clinic run by an expert headache specialist. Patients were evaluated neurologically and underwent imaging and other investigations if indicated to rule out secondary forms of headache. The CM patients were referred from the headache clinic to our Botox clinic.
Patient identification
Both males and females over the age of 18 years with a history (one year or more) of chronic migraine meeting the diagnostic criteria listed in ICHD-III beta [10] were eligible to participate in our study.
Patients were excluded from the study if they had other forms of primary headache disorder, had any neuromuscular junction disorders that might put them at risk with BoNT-A exposure, had uncontrolled systemic disease, abnormal brain or spinal cord pathology contributing to headaches, had concurrent infection at proposed injection sites, or were pregnant, planning for pregnancy or breast-feeding. Patients were also excluded if they were currently using any drugs that might interfere with neuromuscular function, had undergone any pericranial injection for headache within a month time prior to enrollment, or had previously received BoNT-A injection for any reason. Patients were also excluded if had suspected hypersensitivity to BoNTA, or had known or suspected drug or alcohol abuse, or those on narcotics.
Treatment schedule and protocol
On first visit to Botox clinic, the referred patients who satisfy the inclusion and exclusion criteria were enrolled for the study after obtaining an informed consent. They were trained to keep a headache diary thereafter at least 1 month before the injection. They were instructed to note down the headache parameters like type, duration, number of headache days, severity including associated symptoms and number of days on medications and the total amount of pain killers consumed. Treatment with symptomatic medication was allowed “when needed” basis for treatment of break through headaches.
On the second visit, patient’s headache dairies were verified, clarified and documented. First assessment using headache impact test (HIT–6; version 1.1) [11]. It was carried out at baseline to find out the level of limitation of patient’s social life and activities of daily life because of the headache. All the patients were briefed about the number, sites and possible side effects of BoNT-A injection. They were injected with 100 U BoNT-A (Botox; Allergan, Irvine, USA) dissolved in 2 ml of normal saline using 30G insulin needle. Following “fixed sites fixed doses” (FSFD) protocol (8), BoNT-A was injected at 27 sites pericranially - procerus (5U – 1 site), corrugator (10U – 2 sites) frontalis (10U – 2 sites), temporalis (40U – 8 sites), suboccipitalis (10U – 4 sites), trapezius (10U – 4 sites) and neck muscles (15U – 6 sites). The injection was given by the first investigator. Patients were observed for 10-15 minutes following the injection and advised not to rub or massage the injected areas for 24 hours. Patients were informed that the improvement of headache will not be immediate and might take few weeks.
Efficacy and Safety Measures
Patients were evaluated at baseline, and at 4th, 8th and 12th week after the injection. Patients were advised to report any adverse side effects any time during the study period. Investigators also probed for any adverse events by direct questioning and also examining the patients’ headache diaries during each visit. The effectiveness of BoNT-A treatment was evaluated subjectively by noting down total number of headache days, migraine days among them (primary end-point of the study), as well as amount of analgesic medications required (secondary end point) in the past 4 weeks. Health related quality of life (HRQoL) of the headache patient was evaluated objectively by recording HIT-6 at 4th, 8th and 12th week post injection. Patient who had more than 30% reduction in the above parameters was considered as responder.
At the end of 12 weeks all the patients were offered if they are willing to repeat BoNT-A treatment every 3 months and their headache dairy and HIT 6 score were assessed every 3 months during repeated injections.
Data analysis
All the effect related variables were computed during every visit. Simple descriptive statistical tests (mean and standard deviation) are used to describe the numerical values of the sample. Comparisons of values before and after treatment were evaluated using Student’s paired t-test. Ordinal measures (e.g., age, age of onset, medications intake) were compared between responders and non-responders, male and female gender, and those taking regular prophylaxis versus those not on medications using Mann-Whitney U test. The difference between their characteristics was analysed using repeated measures ANOVA to account for both between and within patient variable. A p value of less than 0.05 was considered to be statistically significant.
Results
Demographic and baseline headache characteristics
Overall 40 patients (33 female and 7 male) between 24 and 73 (mean 44) years of age were enrolled for the study. Half of them were professionally active in their job and only 4 out of them were single. Among the 33 females 5 attained menopause and 7 had disorganized menstrual cycle. Average age of onset of headache was 25. 25 years and in one person it started at 3 years of age and in another at 56 years. Most of them, the headache start in frontal (17/40) or temporal (13/40) region. Some of them (17/40) the headache was almost continuous and in most of them it last for 2 to 3 days at a stretch.
Efficacy results
After BoNT-A treatment, there were reduction in all parameters by 35-40% at 4th weeks, 41-45% at 8th weeks and 39-42% at 12th weeks post treatment. At 4th week, 62.5% (25/40) of patients achieved good response while, 37.5% (15/40) indicating no alteration in their headache frequency and severity. At 8th weeks and 12th weeks post treatment 30% (12/40), 25% (10/40) respectively were found to have no response to treatment.
Over the 12 weeks study period, there was a significant reduction in the frequency of headache and the use of acute migraine medications per month at 4th, 8th and 12th weeks compared to baseline. We found significant reduction in the total number of moderate to severe headache days at 4th, 8th and 12th weeks (14.03 ± 6.39, P = .0017; 11.9 ± 6.63, P = .0001; 12.6 ± 7.56, P = .0008, respectively, versus 21.73 ± 6.03 at baseline), number of migraine days at 4th, 8th and 12th weeks post injection. (6.63 ± 3.47, P = .0001; 5.85 ± 3.39, P = .0001; 6.1 ± 3.89, P = .0001, respectively versus of 10.40 ± 2.72 at baseline) and the analgesic consumption at 4, 8 and 12 weeks post injection. (8.78 ± 6.46, P = .00867; 8.53 ± 5.90, P = .006; 10.38 ± 6.71, P = .0429, respectively versus 14.53 ± 136.50 at baseline) (Figure 1). HIT-6 total scores were also significantly improved at 4th, 8th and 12th weeks compared to baseline. (55.83 ± 10.68, P = .0001; 48.05 ± 10.30, P = .00002; 47.64 ± 11.79, P = .000019, respectively versus 74.33 ± 5.24 at baseline) (Figure 1).
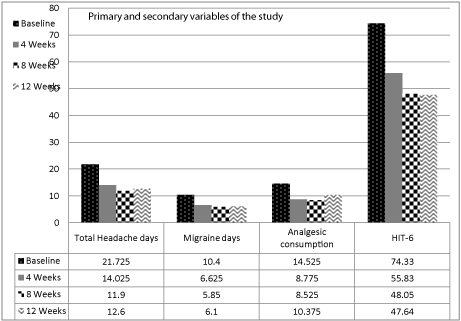
Figure 1: Changes of headache days, migraine days, analgesic consumption
and HIT-6 at baseline, 4, 8 and 12 weeks post treatment.
Baseline demographic characteristics showed no significant differences (p>0.05) between the subgroups of patients, responders (30/40) verses non-responders (10/40), female (33/40) verses male (7/10) and patient not on prophylactic medication (19/40) verses those on medications (21/40) (Figure 2).
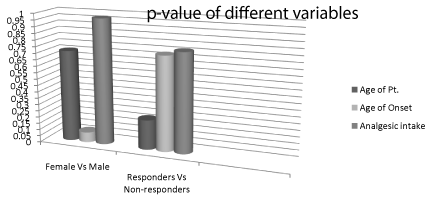
Figure 2: Comparison between subgroup of population based on the
demographic variable and analgesic intake. There are no statistical difference
(p>0.05) between subgroup of population.
Headache Impact Test-6 score of the whole study group showed consistent improvement in the score throughout the study period with a statistically significant reduction (P<0.001) compared to baseline. Comparing the improvement between the subpopulation, there was no significant difference between the groups except the responder versus non-responder group (Figure 3).
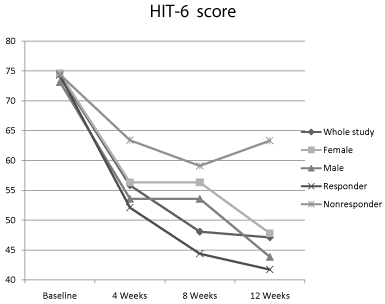
Figure 3: Headache Impact Test-6 score of the whole group and subpopulation
of the study.
Safety and tolerability results
BoNT- A injections were well tolerated, with no significant AEs reported. Only five patients (12.5%) experienced some AEs which were mild and short lasting. Four had cervical pain at injected sites and one had eyebrow asymmetry. There were no reported cases of diplopia, facial expression problems, autonomic or systemic side effects, keratopathy, or idiosyncratic or allergic reactions.
Long term follow up
During the follow up period of 1 year all the patients were assessed clinically in BoNT-A clinic. Out of 30 responders 2 patients improved dramatically after the initial injection and continued to have occasional migraine. They were satisfied with medical treatment without further injection. Among remaining responders 10 patients received BoNT-A 3 times, 8 patients 2 times and 10 patients 1 more time apart from maiden injection. Responders taking repeated injection continued to respond around 60-70% every time. There was significant improvement in total number of headache days and analgesic consumption over 12 months study period (P= 0.04). After the repeat BoNT-A treatment, the number of headache days/month dropped 14.3 ± 8.053 (P=<.0001) after 2nd injection, 13.75 ± 9.432 (P=.0009) after 3rd injection, 14.9 ± 10.545 (P=.0428) after 4th injection, (versus 21.725 ± 6.034 at baseline). Analgesic intake reduced 9.76 ± 7.034 (p=.028) after 2nd injection, 9.33 ± 6.845 (p=.0238) after 3rd injection, 9.5 ± 7.05 (p=.0452) after 4th injection, (versus 14.525 ± 13.508 at baseline). Their HRQoL showed consistent improvement in their social activity by HIT6 score; 54.6 ± 10.754 (p=<.0001) after 2nd injection, 56.25 ± 12.460 (p=<.0001) after 3rd injection, 56.40 ± 13.450 (p=.0002) after 4th injection, (versus 74.325 ± 5.2398 at baseline) (Figure 4).
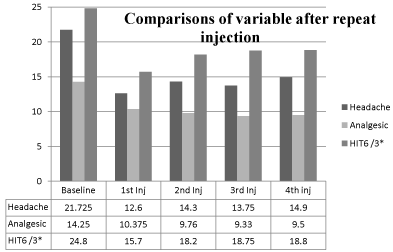
Figure 4: Comparison of primary and secondary endpoints and HIT 6
score after repeated injection of BoNT-A. Primary and secondary endpoints
(average number of headache days and analgesic intake over 30 days
period) and HIT 6 score at the end of the injection period after the 1st injection
(n=40), 2nd injection (n=35), 3rd injection (n=23) and 4th injection (n=13).
*HIT6 x3 = for original score of HIT 6 multiply the value given in the figure by 3.
Though there was no adequate response to initial injection, even the non-responders willing for repeated injection. Among nonresponder 3 received injection 3 times, one patient two times and one patient one time. Most of them respond after repeated injection with reduction in analgesic intake and severity of headache. Only 3 nonresponders were not willing for further injection.
Discussion
In this study, we examined whether BoNT-A prophylaxis of patients who had failed prior trials with other preventive medications resulted in beneficial effects on headache frequency, severity, analgesic consumption and HRQoL. Our cohort included 40 CM patients (33 female and 7 male). Although we inject a lot of headache patients in our BoNT-A clinic, our cohort is small as we strictly included only naïve CM patients without analgesic abuse. In general 50% of chronic headache patients have medications overuse headache [12]. The population-based 1-year prevalence of MOH in different countries ranges from 0.7% to 1.7% [13]. Most of our CM patients attending our BoNT-A clinic also had medication overuse headache, so we excluded them from our study.
We found that up to 75% of patients treated with BoNT-A improved. Assessment of patients at the 4th, 8th and 12th weeks following BoNT-A injection showed significant improvement in all outcome measures compared to baseline. There were improvement in the total number of moderate to severe headache days, number of migraine days and the analgesic consumption. Headache Impact Test-6 total scores was also significantly improved compared to baseline. Our results are consistent with PREEMPT (Phase III Research Evaluating Migraine Prophylaxis Therapy) which enrolled 670 patients with CM and established onabotulinumtoxinA as a safe, well-tolerated, and effective headache prophylactic CM treatment [8].
Most of our patients had continuous dull aching headache throughout the day, so we took only total number of headache days with moderate to severe intensity. During the study period of 12 weeks there was significant reduction in total headache days, migraine days and analgesic consumption, indicating that the vicious cycle of CM had been broken. Treatment of CM is very difficult and reverting continuous nature of CM to an episodic one is highly rewarding [14]. This is what we have achieved in our CM patients. Nevertheless, there is a general feeling that one single treatment session could be insufficient to treat any chronic pain syndrome. However our study has proved that a single injection might be enough to break the cycle of chronic headaches.
HIT-6 is a reliable and valid tool for discriminating headache impact across episodic and chronic migraine. It measures not only the adverse impact of headache on social functioning, role functioning, vitality, cognitive functioning and psychological distress but also measures the severity of headache pain [15]. In our study HIT-6 score proportionally correspond to primary and secondary outcomes.
One of the biggest challenges in our study is choosing the right dose of BoNT-A that will be effective as well as without side effect. Reviewing the literature, there are different studies employing different doses ranging from 75 to 260 U which were found to be effective [16-18]. In one of the double blind placebo controlled study looking at the effectiveness of fixed-site administration of 100 U of BoNT-A in the treatment of CM had demonstrated statistically significant and clinically meaningful (31.0%) decrease in migraine frequency compared to 8.9% decline with placebo group (P <0.001) and also there was less use of pain medications [19]. Moreover injecting a whole vial (100 U) for each patient is not only cost effective and convenient but also avoids wastage as dissolved toxin cannot be used after 24 hours.
There are 2 different technics of BoNT-A injection namely following the pain and fixed dose fixed site protocol. When injected follow-the-pain approach two problems have been reported: first, a poor cosmetic outcome; and second, the headaches often shift to the previously unaffected side [20]. These complications were avoided in our study using fixed dose fixed site protocol which also gave a satisfactory response in controlling headache.
Most published trials have reported minimal to no AEs [8,21- 23]. In a placebo-controlled, double-blind trial, Silberstein et al. [24] found that although no serious AEs occurred, some patients receiving BoNT-A injections experienced transient minor AEs, including blepharoptosis, diplopia, and injection site weakness. In our study, BoNT-A treatment was found to be well-tolerated with the only reported treatment-related AEs being mild injection site pain. Treatment-related AEs were consistent with the known tolerability profile of BoNT-A injected into head and neck muscles, and no newly emerging safety findings were observed in our study. BoNT-A in CM has several advantages over conventional treatment, such as reduced AEs and improved compliance. Mathew and Jaffri [25] reported in patients receiving BoNT-A had fewer AEs leading to discontinuation (7.7%) than patients on topiramate (24.1%), even though both of them showed equal efficacy in controlling CM. Results of our study also confer with other studies that BoNT-A is safe and well-tolerated alternative prophylactic therapy for CM. Although this study demonstrates interesting results, further large scale randomised clinical trials with long-term follow up required to clarify the long term effectiveness of BoNT-A in breaking the cycle of CM.
Long term study showed a constant and cumulative trend of improvement in total number of headache days and analgesic consumption over 12 months study period. A statistically significant decrease in HIT6 score was observed during subsequent injection compare to baseline. The analysis of patients’ diary showed constant trend of improvement in the severity of headache and consistent improvement in HRQoL during this long term follow-up. No serious adverse effect was reported even in this 12 months follow through. Our results are similar to the study of Oterino [23] which included 35 patients, they reported reduction of headache days at least by 50%, frequency of severe attacks by 46% and reduction of analgesic use by 50% with mild adverse events in 6/35 patients. Our results are also consistent with Aurora and his colleague who reported that onabotulinumtoxinA is a safe and effective long-term prophylactic treatment for CM [22].
The limitations of our study are first, it is open-label analysis without blinding or placebo-controlled. Second, the subjects used headache prophylactic medication before enrolment in the study. There may be a doubt whether the benefits might be due to previous headache prophylactic medication, rather than BoNT-A efficacy alone. To recall, these patients were referred from specialized headache clinic after a good trial of medical treatment and the prophylactic medication were failed in control of their headache. So whatever benefit achieved, we feel most likely comes from BoNT-A not due to previous prophylactic medication. Though our study was designed for one-session of BoNT-A injection with 12 weeks followup, however we have followed them for 1 year and injected some of them every 3 months beyond the study period and the effect of BoNT-A showed sustained benefit with every injection and persisted more than we expected.
We conclude that Botulinum toxin A represents a completely new treatment option approved by FDA for patients with chronic migraine syndromes. Our results also demonstrated the superiority of BoNT-A by reducing the frequency of headache resulting in reduced disability and improved functioning, vitality, and overall HRQoL in adults patients with disabling and difficult to manage CM. Although the mechanisms through which BoNT-A exert its beneficial effect remain uncertain, BoNT-A is a welcome addition to the available treatment armamentarium for chronic migraine. This is the appropriate time for clinicians to consider BoNT-A as a treatment option for chronic migraine patients who cannot tolerate or do not benefit from standard therapies.
References
- Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008; 71: 559-566.
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629-808.
- Jensen R, Stovner LJ. Epidemiology and comorbidity of headache. Lancet Neurol. 2008; 7: 354-361.
- Colás R, Muñoz P, Temprano R, Gómez C, Pascual J. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology. 2004; 62: 1338-1342.
- Binder WJ, Brin MF, Blitzer A, Pogoda JM. Botulinum toxin type A (BOTOX) for treatment of migraine. Semin Cutan Med Surg. 2001; 20: 93-100.
- Schulman EA, Lake AE 3rd, Goadsby PJ, Peterlin BL, Siegel SE, Markley HG, et al. Defining refractory migraine and refractory chronic migraine: proposed criteria from the Refractory Headache Special Interest Section of the American Headache Society. Headache. 2008; 48: 778-782.
- Robbins L. Refractory chronic migraine: long-term follow-up using a refractory rating scale. J Headache Pain. 2012; 13: 225-229.
- Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010; 6: 921-936.
- Robertson CE, Garza I. Critical analysis of the use of onabotulinumtoxinA (botulinum toxin type A) in migraine. Neuropsychiatr Dis Treat. 2012; 8: 35-48.
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013; 33: 629-808.
- Bjorner JB, Kosinski M, Ware JE Jr. Calibration of an item pool for assessing the burden of headaches: an application of item response theory to the headache impact test (HIT). Qual Life Res. 2003; 12: 913-933.
- Evers S, Marziniak M. Clinical features, pathophysiology, and treatment of medication-overuse headache. Lancet Neurol. 2010; 9: 391-401.
- Paemeleire K, Crevits L, Goadsby PJ, Kaube H. Practical management of medication-overuse headache. Acta Neurol Belg. 2006; 106: 43-51.
- Pascual J, Cacabelos P. More lessons for the treatment of chronic daily headache. J Headache Pain. 2008; 9: 3-4.
- Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT-6™) across episodic and chronic migraine. Cephalalgia. 2011; 31: 357-367.
- Mathew NT, Frishberg BM, Gawel M, Dimitrova R, Gibson J, Turkel C, BOTOX CDH Study Group. Botulinum toxin type A (BOTOX) for the prophylactic treatment of chronic daily headache: A randomized, double-blind, placebo controlled trial. Headache. 2005; 45: 293-307.
- Silberstein SD, Stark SR, Lucas SM, Christie SN, Degryse RE, Turkel CC, BoNTA-039 Study Group. Botulinum toxin type A for the prophylactic treatment of chronic daily headache: A randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2005; 80: 1126-1137.
- Jakubowski M, McAllister PJ, Bajwa ZH, Ward TN, Smith P, Burstein R. Exploding vs. imploding headache in migraine prophylaxis with Botulinum Toxin A. Pain. 2006; 125: 286-295.
- Evers S, Vollmer-Haase J, Schwaag S, Rahmann A, Husstedt IW, Frese A. Botulinum toxin A in the prophylactic treatment of migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia 2004; 24: 838-843.
- Blumenfeld AM, Dodick DW, Silberstein SD. Botulinum neurotoxin for the treatment of migraine and other primary headache disorders. Dermatol Clin. 2004; 22: 167–175.
- Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010; 30: 804–814.
- Aurora SK, Dodick DW, Turkel CC, DeGryse RE, Silberstein SD, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010; 30: 793–803.
- Oterino A, Ramón C, Pascual J. Experience with onabotulinumtoxinA (BOTOX) in chronic refractory migraine: focus on severe attacks. J Headache Pain. 2011; 12: 235-238.
- Silberstein S, Mathew N, Saper J, Jenkins S. Botulinum toxin type A as a migraine preventive treatment. Headache. 2000; 40: 445–450.
- Mathew NT, Jaffri SF. A double-blind comparison of onabotulinumtoxina (BOTOX) and topiramate (TOPAMAX) for the prophylactic treatment of chronic migraine: a pilot study. Headache. 2009; 49: 1466-1478.