
Research Article
Austin J Clin Neurol 2015;2(7): 1057.
Narcissism Vulnerability as Risk Factor for Alzheimer´s Disease- A Prospective Study
Serrani D*
Department of Psychobiology, National University of Rosario, Argentina
*Corresponding author: Daniel Serrani, Department of Psychobiology, Faculty of Psychology, National University of Rosario, Berutti 250 bis, Rosario, Santa Fe (S2000QBE), Argentina
Received: April 06, 2015; Accepted: June 25, 2015; Published: June 30, 2015
Abstract
Introduction: We examine whether broad factors and specific facets of pathological narcissistic personality are associated with increased risk of incident Alzheimer’s disease (AD) in a prospective cohort study. Participants (n=452) were monitored for up to 36 months from a baseline personality assessment with Pathological Narcissistic Inventory (NPI). The results showed that dementia developed in 159 participants. On average, participants in whom dementia developed were mostly female (p<;.02), slightly older (p<.04), had lower levels of education (p<.03), lower scores on cognitive test (p<.01), higher scores on narcissistic vulnerability scores (p< .01); but no differences in depression score. Adjusted hazard ratios (AHR) and 95% Confidence Interval (CI) for AD were greater for the following subscales of the PNI: Hiding Self [AHR (95% CI) =1.34 (1.18-1.39)], Devaluation [AHR (95% CI) =1.35 (0.65, 0.98)] and Narcissistic Vulnerability [AHR (95% CI) =1.43 (1.34-1.69)]. Also cognitive status and depression showed robust association with incident AD. The current study indicates that narcissistic vulnerability traits are associated with increased risk of AD, with significant hazard ratios.
Keywords: Narcissistic personality; Alzheimer’s disease; Risk of dementia;Depression; Cognitive status
Introduction
Alzheimer’s disease and related dementias (ADRD) represents an increasing burden for patients and caregivers, including physical, emotional, and financial demands for care provision [1-3].
An estimated 5.4 million Americans of all ages had Alzheimer’s disease in 2012, one in eight Americans over age 65, ranging from approximately 53 new cases per 1,000 people aged 65 to 74, to 170 new cases per 1,000 people aged 75 to 84. Global prevalence of dementia is as high as 24 million, and is expected to quadruple by the year 2050. In the US alone, it accounts for an estimate direct health-care costs of $172 billion per year and $210 billion in unpaid care giving [4].
Even when etiological mechanisms underlying this illness remain unknown, both environmental and genetic factors have been proposed as putative causes, beginning years before symptoms became clinically evident. Risk factors for dementia, such as advancing age, family history, genetics, brain reserve, and lifestyle have been proposed [5].
Provided that only modest therapeutic effects have been achieved with currently available pharmacologic disease-modifying treatments for AD, attention has shifted toward prevention or delay of AD onset [6,7].
In a recent systematic review, association of multiple factors with ADRD was assessed, and several potentially modifiable conditions were identified, such as lifestyle, diabetes, hypertension, obesity, smoking, depression, cognitive engagement, physical activity and diet. Surprisingly, personality traits were not addressed [8] despite strong associations between personality and health [9].
Very few studies have evaluated the relationship between premorbid personality, life events and ADRD. For example, early childhood stressful situations could impair elder´s psychosocial adaptation favoring the emergence of ADRD [10].
Previous psychiatric history, including premorbid personality, may predispose subjects to ADRD and its corresponding psychiatric symptoms [11-13] concluding that before Alzheimer’s disease onset, patients were more passive, hostile and less spontaneous. Other authors also reported diminished initiative/growing apathy, relinquishment of hobbies and increased rigidity [14].
Similar results were obtained in studies comparing premorbid and present personality using the NEO-PI instrument [15]. Changes in the five personality domains could be summarized as higher neuroticism, lower extraversion and conscientiousness, and smaller reductions in openness and agreeableness.
In a long term prospective study, higher scores on vulnerability to stress, anxiety, and depression preceded onset of dementia up to 30 years. Those traits were associated with higher ADRD neuropathology at autopsy, and lower resilience to clinical dementia. On the other side, agreeableness, order and competence, as part of a resilient personality, were significantly associated with lower risk or delay of clinical dementia, even when post mortem AD neuropathology was present [16,17]. Those findings were replicated in an independent study [18].
In another research using data from the Religious Orders Study [19] conscientiousness or will and goal directedness were associated with a reduced risk of AD, and to a slower rate of cognitive decline.
Several other studies have acknowledged the association between mid-life personality and incident ADRD [20-24]. Finally, in recent meta-analysis [25] individuals with high neuroticism and low conscientiousness had a threefold increased risk of incident AD, together with self-discipline and depression. Some studies do not support those previous findings, however, and found no relationship between previous personality and risk of dementia [26].
Some authors, indeed, tend to stress the continuity between preand post-morbid personality profiles [27,28]. For those researchers, the personality traits of patients with dementia seemingly reflect adaptive coping models used before onset of dementia. Considering the aforementioned studies, research on personality disorder as a risk factor for dementia usually has been based on psychobiological models [29] or the Five Factor Taxonomy (FFT) of personality [30- 32], and examined relatively homogeneous groups [33].
Given the putative role of education and lifestyle as risk factors for ADRD [34], research should also focus in ethnically heterogeneous cohorts and tap on more clinically oriented personality clusters. In that sense, subjects with personality disorders and associated neurobiological vulnerabilities, such as Borderline, Narcissistic or Antisocial Personality Disorder [35,36] could be prone to develop neurodegenerative diseases such as dementia in old age.
However, only few case reports are available to support this hypothesis [37-39] without corresponding epidemiological data. With regard to Narcissistic Personality Disorder, the most consistent findings have been a positive correlation with negative emotionality, aggressiveness, psychoticism and extraversion; a negative correlation with agreeableness and conscientiousness36, and inconsistent correlation with introversion and low positive emotionality.
A substantial degree of variability in convergence between measures of narcissism grandiosity and vulnerability and the domains of the FFT still persist [40], suggesting that narcissistic personality as risk factor for dementia should be studied in its own, and not simply associating it with the FFT results [41]. As most of the evidence on risk factors for ADRD has been based on case-control studies, longitudinal studies may represent a better design to identify risk factors for development of ADRD of affected individuals. They avoid the need to obtain exposure data from proxy respondents as individuals affected with ADRD may be unable to provide reliable data.
In conclusion, the objective of this research was to investigate the role of previous pathological narcissistic personality disorder as a risk factor for ADRD in a Spanish-speaking population using a longitudinal study. Ultimately, we hope that these data could aid in two ways: first, developing clinically useful risk markers for predicting ADRD, which, in turn, would pave the way for dementia prevention strategies; second, studying the risk posed by personality could yield insights about the etiology of ADRD [42-44].
Materials and Methods
Sample size
Based on an age-standardized prevalence for ADRD of 4.4% [45- 47], a relative risk (RR) of 1.19 in relation to premorbid health status, an alpha risk of 5%, a power of 95% and an expected drop-out rate of 20% over 36 months, 452 patients were selected.
Study design
The research was based on a prospective cohort study of Spanishspeaking subjects with personality disorders who resided in the community, and received psychiatric treatment at a community mental health center. Inclusion criteria included age 65-75 years, availability of a proxy respondent, preserved ability to complete clinical and neuropsychological evaluations, sign informed consent, and exhibit normal cognitive performance and no significant morbidity. Exclusion criteria included severe visual or hearing impairment, dementia, idiopathic Parkinson’s disease, liver disease, alcoholism, known terminal illness, hospitalization for depression within the last year or having received electroconvulsive therapy within the prior decade, current treatment with cholinesterase inhibitors, anti-Parkinson medications, tricyclic antidepressants, antipsychotics or other medications with significant psychotropic or central cholinergic effects. Patients were enrolled and interviewed from March 2009 to March 2010. At baseline, patients underwent a clinical, neuropsychological and psychiatric examination, and a brain MRI. Patients were followed up over a period of 36 months, at 6 months intervals. Informed written consent was obtained from subject and caregiver at enrollment and before baseline assessment took place. The study protocol was reviewed and approved by the local institutional review board. All procedures were in accordance with the declaration of Helsinki. The sample was middle-class, most subjects were female (63 percent). Only 2 subjects with missing data, and 7 subjects who moved or declined to return for follow-up were excluded. After that, 452 subjects remained available for the study.
Measures
Cognitive impairment was assessed using the Modified Mini- Mental State Exam (normal values ≥78) [48]. The 3MSE is based on the Mini-Mental State Exam and offers a more graded scoring.
Depression was assessed using the Geriatric Depression Scale (Short Form) [49] which has shown good psychometric properties. A score of 0 to 5 is normal. A score greater than 5 suggests depression.
Diagnosis of dementia was made using the informant/subject structured interview based on the Clinical Dementia Rating Scale (CDR) (normal values ≤ 0.5) [50] and the Blessed Information Memory Concentration scale (normal values ≤ 4) [51]. This last test exhibits a high test–retest reliability (0.86), and correlate closely with the stages of Alzheimer’s disease [52].
Narcissistic Pathological Personality was assessed with the Pathological Narcissism Inventory (PNI) [53] which is a 52-item multidimensional self-report measure of pathological narcissism. Respondents are asked to use a 6-point scale ranging from 0 (not at all like me) to 5 (very much like me) to rate each item. It consists of seven subscales that measure different characteristics of pathological narcissism: Contingent Self-Esteem (CSE), Exploitativeness (EXP), Self-Sacrificing Self-Enhancement (SSSE), Hiding the Self (HS), Grandiose Fantasy (GF), Devaluing (DEV), and Entitlement Rage (ER). Because of the variability in scale length, mean scores are used instead of sums to allow for easy comparison across scales. Two higher order factors encompassing these 7 subscales, Narcissistic Grandiosity and Narcissistic Vulnerability are also scored.
Statistical analysis
Data were analyzed using Stata (Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). Continuous variables were compared with use of either an independent-samples t-test or the Mann–Whitney U test, and categorical variables were compared with use of the Pearson chi-square test. The association between personality and risk of dementia was assessed using cox proportionalhazards regression analysis to estimate hazard ratios, with 95 percent confidence intervals. The time to an event was defined as time evolved from enrollment to the date when a diagnosis of dementia was issued. Censoring was based on study ending, time of death or follow-up refusal. All multivariate models included following covariates: age at enrollment, sex, educational level (high school vs. college-level education) and base-line scores of 3MSE and geriatric depression scale. Graphical (log-log plots) and analytical (Schoenfeld residuals) methods were used to test the proportional hazards assumption for each analysis; the assumption was supported in every case. Analyses also examined interactions between each of the PNI subscales and other covariates in the model. Finally, analyses explored curvilinear relationships between the PNI subscales and dementia risk, by including each factor together with a squared term for each factor.
Results
During 1104 person-years of follow-up (median follow-up, 2.7 years), dementia developed in 159 participants (Alzheimer’s disease in 112 (70%), vascular dementia in 37 (23%), mixed dementia in 9 (1.5%), and other types of dementia in 1 (0.5%). By the end of the study period, no subjects had died and 7 participants had dropped out (follow-up, 2.7±1.1 years). On average, participants in whom dementia developed were mostly female (t=3.7, p<.02), slightly older (t=4.9, p<.04), had lower levels of education (t=5.2, p<.03), lower scores on cognitive test (t=4.9, p<.01), higher scores on narcissistic vulnerability scores (t=8.7, p< .01); but no differences in depression score (Table 1).
Variable
no incident dementia
(n=259)
incident dementia
(n=159)
p value
Age (yrs.)
73.2± 2.9
75.1± 3.0
.04
Gender (female) (%)
149 (57%)
135 (85%)
.02
Duration of follow up (months)
32.1±4.1
33.8±3.9
.50
High school education (n/%)
220 (85%)
112 (71%)
.03
3MSE
2.45±0.3
1.02±0.4
.01
GDS
5.33±2.1
5.71±1.8
.35
Narcissistic grandiosity
3.21±1.1
2.87±1.9
.09
Narcissistic vulnerability
2.08±1.5
4.56±2.1
.01
CSE
2.91±0.3
2.07±0.6
.57
EXP
2.76±0.5
2.79±0.4
.04
SSSE
2.19±1.0
3.16±1.1
.05
HS
1.87±0.3
4.01±1.7
.01
GF
2.08±0.9
2.33±1.6
.23
DEV
1.26±1.5
4.07±2.3
.01
ER
2.03±1.8
3.47±1.5
.02
Plus–minus values are means ±SD. P values for scales and tests were calculated by the Mann–Whitney U test. 3MSE rating range from 1 to 3, with higher scores indicating better cognitive function; scores on the GDS (Geriatric Depression Scale) range from 0 to 15, with higher scores indicating greater depression; scores on the PNI (pathological personality Inventory) range from 0 to 312, with higher scores indicating more pathological personality traits. Contingent Self-Esteem (CSE), Exploitativeness (EXP), Self-Sacrificing Self-Enhancement (SSSE), Hiding the Self (HS), Grandiose Fantasy (GF), Devaluing (DEV), and Entitlement Rage (ER).
Table 1: Baseline Demographic Characteristics of Participants by final dementia status.
The covariates between pathological narcissistic personality subscales along with depression and cognitive scores are observed in Table 2. More robust correlations were observed between depression scores and narcissistic vulnerability and devaluating the self. Also correlations between other covariates were found, such as narcissistic vulnerability and hiding and devaluating the self.
3MSE
GDS
NG
NV
CSE
EXP
SSSE
HS
GF
DEV
ER
3MSE
48.6
48.4
-38.4
56.9
46.2
52.5
45.3
53.9
-34.1
-41.2
GDS
-35.6
67.3
31.2
37.9
-32.5
-66.8
37.9
-57.9
-47.0
NG
-19.9
51.1
49.3
58.4
-31.4
51.8
-38.6
50.8
NV
35.6
-33.9
38.5
66.2
20.5
69.2
28.9
CSE
51.5
40.2
21.4
49.5
28.5
47.1
EXP
38.5
-27.4
41.3
20.5
48.8
SSSE
-48.9
54.1
39.2
38.9
HS
-65-2
41.0
-51.3
GF
-46.1
-50.1
DEV
25.8
Contingent Self-Esteem (CSE), Exploitativeness (EXP), Self-Sacrificing Self-Enhancement (SSSE), hiding the Self (HS), Grandiose Fantasy (GF), Devaluing (DEV), and Entitlement Rage (ER), Mini-mental state examination 3 items (3MSE), geriatric Depression Scale (GDS)
Table 2: Correlations Among personality subscales, depression and cognition (N=452).
Table 3 shows the adjusted hazard ratios (AHR) and 95% confidence intervals (CI) for AD for each of the subscales of PNI. Each subscale is first reported when entered alone in the model, and then associated with the other subscales. Of the confounders, only age and education were independently associated with AD risk. Older participants [AHR (95% CI) =1.32 (1.18-1.36)] were at greater risk of developing AD over the follow-up period. In addition, AD risk was greater among participants with higher scores in HS, DEV and NV and lower in GF and NG. When the 3MSE was added to the fixed model, participants higher in NV, DEV and HS remained at greater risk. The AHRs for NV, DEV AND HS were slightly greater than corresponding values for fixed model. Whereas the figures for NV were unaffected by adding other subscales to the model, NG was no longer statistically significant. People who scored lower on the 3MSE at study entry were more likely to develop AD, AHR (95%CI) = 1.84 (1.71-1.90). When GDS was added to the model, NV, HS and DEV remained associated with higher AD risk, and AHRs scores were slightly greater than those in the fixed model. The positive association between GDS scores and AD risk was not as large as the effect of 3MSE [AHR (95%CI) =1.18 (1.12-1.23)].In sum, AD risk was associated with higher HS [AHR (95% CI) =1.34 (1.18-1.39)], DEV [AHR (95% CI) =1.35(0.65, 0.98)] and NV [AHR (95% CI) =1.43 (1.34-1.69)].
Fixed
Model 2
Model 3
Predictor
Age, gender, education
Base+3MSE
Base+GDS
CSE
1.14 (1.09-1.19)
1.16 (1.11-1.24)
1.27 (1.20-1.32)
CSE-PNI
1.06 (1.01-1.14)
1.05 (1.01-1.11)
1.12 (1.04-1.20)
EXP
.98 (.73-1.12)
.92 (.85-1.21)
.93 (.67-1.11)
EXP-PNI
.90 (.88-1.17)
.87 (.65-1.13)
.89 (.56-1.21)
SSSE
1.00 (.78-1.09)
1.01 (.87-1.10)
1.03 (.73-1.14)
SSSE-PNI
.98 (.67-1.13)
.99 (.56-1.16)
.87 (.78-1.16)
HS
1.34 (1.18-1.39)
1.45 (1.37-1.49)
1.42 (1.36-1.49)
HS-PNI
1.44 (1.40-1.49)
1.53 (1.48-1.59)
1.58 (1.53-1.62)
GF
1.01 (.84-1.12)
0.98 (.76-1.09)
1.02 (.89-1.10)
GF-PNI
.97 (.86-1.13)
.87 (.75-1.15)
.86 (.56-1.08)
DEV
1.35 (1.21-1.57)
1.43 (1.31-1.56)
1.53 (1.32-1.78)
DEV-PNI
1.32 (1.23-1.48)
1.21 (1.15-1.37)
1.31 (1.24-1.57)
ER
1.02 (.85-1.15)
.98 (.56-1.12)
1.03 (.89-1.10)
ER-PNI
1.21 (1.16-1.31)
1.12 (1.03-1.26)
1.10 (1.01-1.21)
NV
1.43 (1.34-1.69)
1.52 (1.34-1.68)
1.78 (1.56-1.97)
NV-PNI
1.45 (1.32-1.59)
1.64 (1.45-1.87)
1.67 (1.40-1.81)
NG
.98 (.67-1.10)
.76 (.53-.99)
.86 (.56-1.03)
NG-PNI
.95 (.78-1.18)
.87 (.56-.99)
.79 (.45-.89)
Contingent Self-Esteem (CSE), Exploitativeness (EXP), Self-Sacrificing Self-Enhancement (SSSE), Hiding the Self (HS), Grandiose Fantasy (GF), Devaluing (DEV), and Entitlement Rage (ER), Pathological Narcissistic Inventory (PNI)
Table 3: Adjusted Hazard Ratios (AHR) for Alzheimer’s disease (N=159).
The Kaplan-Meier probability of developing AD over the followup is presented in Figure 1. Relative to the low risk group, the AD HR for high risk group was 2.12 (95% CI =1.79-3.89), and with full adjustment, including age, gender, education level, GDS and 3MSE, HR was 2.07(95% CI=1.18-3.75). Supplementary and sensitivity analyses revealed a significant interaction between NV subscale and DEV, HS and SSSE covariates. No gender differences were found in the relationship between NV and AD risk. Only squared NV subscale scores were significant.
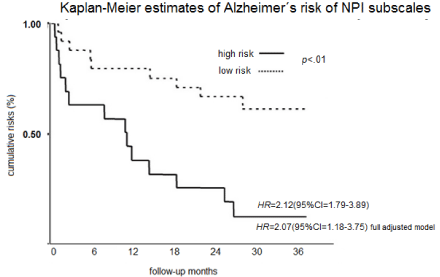
Figure 1: Kaplan-Meier estimates of AD risk of NPI sub-scales.
Discussion
In this study a clear association was disclosed between elders with high scores in pathological narcissism and greater risk for AD. For NV, HS and MOV, findings were somewhat comparable when entered alone or in conjunction with the other NPI subscales. Results remained significant even after depression was inserted in the model, and the same could be said regarding cognitive function. The predictive value of NG was not apparent when all subscales were entered simultaneously, suggesting that NV is an independent risk factor per se. Some authors [54] further distinguish between grandiosity and vulnerability narcissism, the first associated with overt expressions of grandiose fantasies, arrogance and self-entitlement, the latter with themes of fragility, depletion and feelings of inadequacy. Although patients may exhibit both aspects of narcissistic personality, others could probably express only one of those facets much of the time, for example narcissistic vulnerability, being more at risk.
Results for pathological narcissism appear quite robust; however the influence of other variables which probably intervene in the pathway from pathological narcissism to AD, such as comorbid pathological conditions, should be taken into account in future investigations, as they may alter the present results. These findings must be added to the growing literature acknowledging the deleterious effects on cognitive function of dysfunctional coping mechanisms observed in pathological narcissistic personality together with HPA axis dysregulation [55-57]. It is also likely that poor decision making due to perceived high self-efficacy and risk taking [58], and compromised self-care with excessive dieting and over-exercising [59,60] increase risk for AD together with other adverse health outcomes [61]. It is also worth to examine the association between personality disorders, particularly vulnerable narcissism, and cognitive deterioration [62], suggesting that pathological narcissists should benefit from socially embedded, spontaneous cognitive activities with varied content, such as daydreams, fantasies and games [63]. However, data provided in the present study do not allow us to draw any conclusions regarding which type of cognitive activity could play a protective role for dementia risk. In this study a sound relationship was observed between low education and high risk for dementia, which has been mentioned in several studies [64,65]. Given that personality disorders, such as narcissism, have been tied to low education and social outcomes [66,67], pathological narcissism traits could play a mediating role in this association. In that sense, reducing the influence of narcissistic vulnerability and exposing the subject to more stimulant and healthy cognitive and social activities could aid in reducing the risk of AD. Some limitations to this study must be acknowledged: first, selective loss of subjects with specific personality profiles may have influenced the observed relationships. As pathological personality traits are associated with lower health outcomes and greater mortality rates [68], healthy survivor effects would underestimate the associations between those traits and dementia. Second, given that changes in personality may predate clinical onset of dementia [69], it is possible that at time of recruitment the presence of incipient Alzheimer’s disease may alter personality diagnosis. Third, as availability of proxy respondents was set as an inclusion criterion, lack of them may pose implications for the cohort’s sampling strategy.
Conclusion
Our findings suggest that elevated scores in narcissistic vulnerability traits may be an important risk factor for dementia. These findings have importance on the design and implementation of preventive strategies for dementia, and in the conceptualization of the multifactorial etiology of Alzheimer’s disease.
References
- Takizawa C, Thompson PL, van Walsem A, Faure C, Maier WC. Epidemiological and economic burden of Alzheimer's disease: a systematic literature review of data across Europe and the United States of America. J Alzheimers Dis. 2015; 43: 1271-1284.
- Haro JM, Kahle-Wrobleski K, Bruno G, Belger M, Dell’Agnello G, Dodel R, et al. Analysis of burden in caregivers of people with Alzheimer’s disease using self-report and supervision hours. J Nutr Health Aging. 2014; 18: 677-684.
- Hazzan AA, Shannon H, Ploeg J, Raina P, Oremus M. Association between Caregiver Quality of Life and the Care Provided to Persons with Alzheimer’s Disease: Systematic Review. Syst Rev. 2013; 13: 2-17.
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013; 368: 1326-1334.
- Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014; 88: 640-651.
- Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, et al. Clinical trials and late-stage drug development for Alzheimer's disease: an appraisal from 1984 to 2014. J Intern Med. 2014; 275: 251-283.
- Lin PJ, Yang Z, Fillit HM, Cohen JT, Neumann PJ. Unintended benefits: the potential economic impact of addressing risk factors to prevent Alzheimer's disease. Health Aff (Millwood). 2014; 33: 547-554.
- Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES Jr, Cox NJ, et al. NIH state-of-the-science conference statement: Preventing Alzheimer's disease and cognitive decline. NIH Consens State Sci Statements. 2010; 27: 1-30.
- Kern ML, Friedman HS. Do conscientious individuals live longer? A quantitative review. Health Psychol. 2008; 27: 505-512.
- Wilson RS, Krueger KR, Arnold SE, Barnes LL, Mendes de Leon CF, Bienias JL, et al. Childhood adversity and psychosocial adjustment in old age. Am J Geriatr Psychiatry. 2006; 14: 307-315.
- Cooper B, Holmes C. Previous psychiatric history as a risk factor for late-life dementia: a population-based case-control study. Age Ageing. 1998; 27: 181-188.
- Chatterjee A, Strauss ME, Smyth KA, Whitehouse PJ. Personality changes in Alzheimer's disease. Arch Neurol. 1992; 49: 486-491.
- Petry S, Cummings JL, Hill MA, Shapira J. Personality alterations in dementia of the Alzheimer type. Arch Neurol. 1988; 45: 1187-1190.
- Bózzola FG, Gorelick PB, Freels S. Personality changes in Alzheimer's disease. Arch Neurol. 1992; 49: 297-300.
- Costa PT, McCrae RR. The NEO personality inventory manual. Odessa, Florida: Psychological Assessment Resources. 1985.
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004; 3: 343-353.
- Paillard-Borg S, Fratiglioni L, Xu W, Winblad B, Wang HX. An active lifestyle postpones dementia onset by more than one year in very old adults. J Alzheimers Dis. 2012; 31: 835-842.
- Wilson RS, Begeny CT, Boyle PA, Schneider JA, Bennett DA. Vulnerability to stress, anxiety, and development of dementia in old age. Am J Geriatr Psychiatry. 2011; 19: 327-334.
- Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res. 2012; 9: 628-645.
- Wang HX, Karp A, Herlitz A, Crowe M, Kåreholt I, Winblad B, et al. Personality and lifestyle in relation to dementia incidence. Neurology. 2009; 72: 253-259.
- Balsis S, Carpenter BD, Storandt M. Personality change precedes clinical diagnosis of dementia of the Alzheimer type. J Gerontol B Psychol Sci Soc Sci. 2005; 60: P98–P101.
- Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, et al. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology. 2005; 64: 380-382.
- Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer's disease in old age. Neuroepidemiology. 2006; 27: 143-153.
- Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Arch Gen Psychiatry. 2007; 64: 1204-1212.
- Terracciano A, Sutin AR, An Y, O'Brien RJ, Ferrucci L, Zonderman AB, et al. Personality and risk of Alzheimer's disease: new data and meta-analysis. Alzheimers Dement. 2014; 10: 179-186.
- Ramakers IH, Honings ST, Ponds RW, Aalten P, Köhler S, Verhey FR, et al. The Effect of Psychological Distress and Personality Traits on Cognitive Performances and the Risk of Dementia in Patients with Mild Cognitive Impairment. J Alzheimers Dis. 2015.
- Pocnet C, Rossier J, Antonietti JP, von Gunten A. Personality traits and behavioral and psychological symptoms in patients at an early stage of Alzheimer's disease. Int J Geriatr Psychiatry. 2013; 28: 276-283.
- Kolanowski AM, Strand G, Whall A. A pilot study of the relation of premorbid characteristics to behavior in dementia. J Gerontol Nurs. 1997; 23: 21-30.
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry. 1993; 50: 975-990.
- Crowe M, Andel R, Pedersen NL, Fratiglioni L, Gatz M. Personality and risk of cognitive impairment 25 years later. Psychol Aging. 2006; 21: 573-580.
- Digman JM. Personality structure: Emergence of the five-factor model. Annual Review of Psychology. 1990; 41: 417–440.
- Donnellan MB, Oswald FL, Baird BM, Lucas RE. The mini-IPIP scales: tiny-yet-effective measures of the Big Five factors of personality. Psychol Assess. 2006; 18: 192-203.
- Gatz M, Mortimer JA, Fratiglioni L, Johansson B, Berg S, Andel R, et al. Accounting for the relationship between low education and dementia: a twin study. Physiol Behav. 2007; 92: 232-237.
- McCloskey MS, Phan KL, Coccaro EF. Neuroimaging and personality disorders. Curr Psychiatry Rep. 2005; 7: 65-72.
- Schulze L, Dziobek I, Vater A, Heekeren HR, Bajbouj M, Renneberg B, et al. Gray matter abnormalities in patients with narcissistic personality disorder. J Psychiatr Res. 2013; 47: 1363-1369.
- Helmes E, Steward L. The case of an aging person with borderline personality disorder and possible dementia. Int Psychogeriatr. 2010; 22: 840-843.
- Salzbrenner LS, Brown J, Hart G, Dettmer EJ, Williams LR, Ormeno LM, et al. Frontotemporal dementia complicated by comorbid borderline personality disorder: a case report. Psychiatry (Edgmont). 2009; 6: 28-31.
- Poletti M, Bonuccelli U. From narcissistic personality disorder to frontotemporal dementia: a case report. Behav Neurol. 2011; 24: 173-176.
- Saulsman LM, Page AC. The five-factor model and personality disorder empirical literature: A meta-analytic review. Clin Psychol Rev. 2004; 23: 1055-1085.
- Samuel DB, Widiger TA. A meta-analytic review of the relationships between the five-factor model and DSM-IV-TR personality disorders: A facet level analysis. Clin psychol rev. 2008; 28: 1326-1342.
- Cain NM, Pincus AL, Ansell EB. Narcissism at the crossroads: phenotypic description of pathological narcissism across clinical theory, social/personality psychology, and psychiatric diagnosis. Clin Psychol Rev. 2008; 28: 638-656.
- Krueger RF, Caspi A, Moffitt TE. Epidemiological personology: the unifying role of personality in population-based research on problem behaviors. J Pers. 2000; 68: 967-998.
- Osborne H, Simpson J, Stokes G. The relationship between pre-morbid personality and challenging behaviour in people with dementia: A systematic review. Aging Ment Health. 2010; 14: 503-515.
- Mendez Rubio M, Antonietti JP, Donati A, Rossier J, von Gunten A. Personality traits and behavioural and psychological symptoms in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2013; 35: 87-97.
- Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000; 54: S4-9.
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007; 29: 125-132.
- Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009; 11: 111-128.
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987; 48: 314-318.
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986; 5: 165-173.
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997; 9 Suppl 1: 173-176.
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968; 114: 797-811.
- Grober E, Dickson D, Sliwinski MJ, Buschke H, Katz M, Crystal H, et al. Memory and mental status correlates of modified Braak staging. Neurobiol Aging. 1999; 20: 573-579.
- Pincus AL, Ansell EB, Pimentel CA, Cain NM, Wright AG, Levy KN. Initial construction and validation of the Pathological Narcissism Inventory. Psychol Assess. 2009; 21: 365-379.
- Pincus AL, Lukowitsky MR. Pathological narcissism and narcissistic personality disorder. Annu Rev Clin Psychol. 2010; 6: 421-446.
- Campbell WK, Goodie AS, Foster JD. Narcissism, confidence, and risk attitude. Journal of Behavioral Decision Making. 2004; 17: 297–311.
- Foster JD, Shenesey JW, Goff JS. Why do narcissists take more risks? Testing the roles of perceived risks and benefits of risky behaviors. Personality and Individual Differences. 2009; 47: 885–889.
- Gleason ME, Weinstein Y, Balsis S, Oltmanns TF. The enduring impact of maladaptive personality traits on relationship quality and health in later life. J Pers. 2014; 82: 493-501.
- Byrne KA, Worthy DA. Do narcissists make better decisions? An investigation of narcissism and dynamic decision-making performance. Personality and Individual Differences. 2013; 55: 112-117.
- Zerach G. The associations between pathological narcissism, alexithymia and disordered eating attitudes among participants of pro-anorexic online communities. Eat Weight Disord. 2014; 19: 337-345.
- Miller KJ, Mesagno C. Personality traits and exercise dependence: Exploring the role of narcissism and perfectionism. International Journal of Sport and Exercise Psychology 2014; 12: 368-381.
- Hill EM. The role of narcissism in health-risk and health-protective behaviors. J Health Psychol. 2015;.
- Duberstein PR, Chapman BP, Tindle HA, Sink KM, Bamonti P, Robbins J, et al. Personality and risk for Alzheimer's disease in adults 72 years of age and older: a 6-year follow-up. Psychol Aging. 2011; 26: 351-362.
- Kremen WS, Lachman ME, Pruessner JC, Sliwinski M, Wilson RS. Mechanisms of age-related cognitive change and targets for intervention: social interactions and stress. J Gerontol A Biol Sci Med Sci. 2012; 67: 760-765.
- Meng X, D'Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One. 2012; 7: e38268.
- Ciarrochi J, Heaven PC, Davies F. The impact of hope, self-esteem, and attributional style on adolescents’ school grades and emotional well-being: A longitudinal study. J Res Personality. 2007; 41: 1161-1178.
- Hampson SE, Goldberg LR, Vogt TM, Dubanoski JP. Forty years on: teachers' assessments of children's personality traits predict self-reported health behaviors and outcomes at midlife. Health Psychol. 2006; 25: 57-64.
- Trzesniewski KH, Donnellan MB, Moffitt TE, Robins RW, Poulton R, Caspi A. Low self-esteem during adolescence predicts poor health, criminal behavior, and limited economic prospects during adulthood. Dev Psychol. 2006; 42: 381-390.
- Jokela M, Pulkki-Råback L, Elovainio M, Kivimäki M. Personality traits as risk factors for stroke and coronary heart disease mortality: pooled analysis of three cohort studies. J Behav Med. 2014; 37: 881-889.
- Hock RS, Lee HB, Bienvenu OJ, Nestadt G, Samuels JF, Parisi JM, et al. Personality and cognitive decline in the Baltimore Epidemiologic Catchment Area follow-up study. Am J Geriatr Psychiatry. 2014; 22: 917-925.