
Case Report
Austin J Clin Neurol 2015;2(7): 1064.
Brain MRI Lesions in Neuromyelitis Optica Spectrum Disorders
Monica B Dhakar1,2, Sanjeev Sivakumar1,2, Navid Seraji-Bozorgzad¹, Omar Khan¹* and Pratik Bhattacharya¹
¹Department of Neurology; Wayne State University School of Medicine, Detroit, MI
²The Detroit Medical Center, Detroit
*Corresponding author: Omar Khan, Department of Neurology, Wayne State University School of Medicine, 4201 St Antoine; 8D-University Health Center, Detroit, MI 48201,
Received: April 20, 2015; Accepted: June 15, 2015; Published: June 29, 2015
Abstract
A 22-year-old woman presented with vomiting, vertigo, and difficulty in walking. Brain MRI scans showed large T2 weighted lesions in the corpus callosum and periventricular white matter. Initial MRI of the cervical spine was unremarkable, but re-evaluation four months later revealed a longitudinally extensive lesion in the cervical cord and positive serum NMO IgG antibody. Brain MRI abnormalities are not uncommon in NMO Spectrum Disorders (NMOSD) and may precede the occurrence of optic neuritis or transverse myelitis. It is important to recognize brain MRI abnormalities that may assist in investigating NMOSD, leading to timely diagnosis and therapeutic intervention.
Keywords: Neuromyelitis Optica Spectrum Disorders; Multiple sclerosis; Devic’s syndrome; All demyelinating disease (CNS); Transverse myelitis
Introduction
Neuromyelitis optica (NMO) is an inflammatory demyelinating disorder of the central nervous system, characterized by relapses of optic neuritis and transverse myelitis. Recent studies have shown that NMO is a pathologically distinct clinical entity and also introduced the clinical syndrome of NMO Spectrum Disorder (NMOSD). For years, the diagnosis of NMO was supported by the absence of brain Magnetic resonance image (MRI) lesions. However, the 2006 diagnostic criteria for NMO were revised to include brain MRI lesions that were not consistent with the diagnosis of multiple sclerosis (MS) [1]. This revision was based on the studies that reported brain lesions in NMO or NMOSD [2]. These include lesions that appear to be tumefactive as well as lesions suggestive of acute disseminated encephalomyelitis (ADEM). We report a case of an African-American patient in whom the tumefactive cerebral and peri-ependymal lesions preceded the onset of transverse myelitis who was found to be seropositive for NMO IgG antibody.
Case Report
A 22-year-old previously healthy African-American woman presented with nausea, vomiting, and vertigo that persisted for one week. Brain MRIs can showed large T2 weighted hyper intense tumefactive lesions involving the corpus callosum, bilateral cerebral white matter, right pons, middle cerebellar peduncles and right cerebellar hemisphere with minimal peripheral enhancement surrounding these lesions (Figure 1). MRI of cervical spine at the time of her initial presentation was unremarkable. The serum NMO IgG assay was not performed at this time. She was treated with intravenous methyl prednisolone (IVMP) for 5 days with a presumed diagnosis of ADEM and underwent rehabilitation. One month later, she developed weakness of the right upper and lower extremities. Brain MRI showed persistent but no new T2 lesions. She was treated with IVMP and plasma exchange for recurrent ADEM with minimal improvement. Four months from the time of initial presentation, she developed neck pain, urinary retention, numbness, and progressive weakness affecting all four extremities. Cervical spine MRI revealed a longitudinally extensive T2 hyperintense lesion extending from the medulla down to T1 thoracic spinal cord (Figure 1), while brain MRI showed interval progression with new T2 lesions. Serum NMOIgG antibody was positive and she was diagnosed with sero-positive NMOSD.
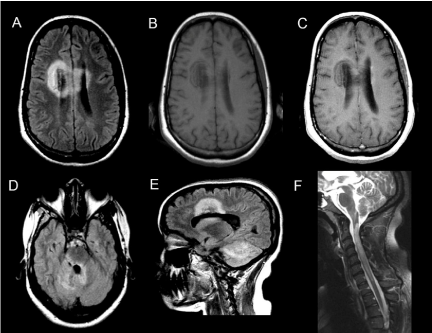
Figure 1: Axial FLAIR image demonstrating lesions involving the anterior
corpus callosum spanning both cerebral hemispheres (A) and periaqueductal
region (D), right lateral pons, bilateral middle cerebellar peduncles and
right cerebellar hemisphere (E). Pre and post contrast T1-weighted images
showing minimal peripheral enhancement (B and C). (F) Sagittal T2-weighted
spinal cord image demonstrating longitudinally extensive lesion extending
from the medulla though the entire cervical cord 4 months after initial
presentation.
Discussion
Brain abnormalities are found in approximately 60% of patients with NMO and NMOSD, which have been well described in Asian population [2]. Most of these lesions are non-specific and can at times even fulfill the ‘Barkhof criteria’ for diagnosis for MS [3]. Most commonly, the lesions tend to cluster around the peri-ependymal regions such as hypothalamus, third, and fourth ventricles, as seen in our patient [4]. These areas correspond to the locations for high expression of aquaporin 4 (AQP4) channels that are concentrated at astrocytes in the blood brain barrier. Emerging studies in the pathogenesis of NMO and NMOSD have shown that that antibodies to AQP4 are pathogenic and highly specific to these disorders [5]. Large tumefactive lesions involving corpus callosum, similar to our case, have been described in prior studies [4, 6]. In one study, a similar cerebral tumefactive lesion became cavitary and subsequent brain autopsy demonstrated pathology similar to cavitary lesions typically found in the spinal cord of NMO patients [7]. The lack of gadolinium enhancement or thin cloud-like enhancement has been reported consistently in these studies and helps differentiate NMOSD from MS or ADEM [6]. Most of the lesions described in these studies occurred during the course of disease.
Our patient developed brain lesions prior to the onset of transverse myelitis by several months. It is important to recognize these lesions and investigate for NMO due to its aggressive nature and therapeutic considerations distinct from MS and ADEM. In conclusion, our case demonstrates that brain lesions are not only common in NMOSD but may have characteristic features such as peri-ependymal location, large tumefactive appearance without significant gadolinium enhancement, distinguishing it from MS and ADEM. Furthermore, brain lesions in NMSOD may precede the onset of transverse myelitis by several months and may aid in early diagnosis.
References
- Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006; 66: 1485-1489.
- Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol. 2006; 63: 390-396.
- Matthews L, Marasco R, Jenkinson M, Kuker W, Luppe S, Leite MI, et al. Distinction of seropositive NMO spectrum disorder and MS brain lesion distribution. Neurology. 2013; 80: 1330-1337.
- Kim W, Park MS, Lee SH, Kim SH, Jung IJ, Takahashi T, et al. Characteristic brain magnetic resonance imaging abnormalities in central nervous system aquaporin-4 autoimmunity. Multiple sclerosis. 2010; 16: 1229-1236.
- Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004; 364: 2106-2112.
- Ikeda K, Ito H, Hidaka T, Takazawa T, Sekine T, Yoshii Y, et al. Repeated non-enhancing tumefactive lesions in a patient with a neuromyelitis optica spectrum disorder. Internal medicine. 2011; 50: 1061-1064.
- Nakamura M, Endo M, Murakami K, Konno H, Fujihara K, Itoyama Y. An autopsied case of neuromyelitis optica with a large cavitary cerebral lesion. Multiple sclerosis. 2005; 11: 735-738.