
Case Report
Austin J Clin Neurol 2015; 2(9): 1074.
Central Pontine Myelinolysis in Patient with Normal Serum Sodium Levels, System Alcohol Use and Malnutrition- A Case Report
I Staikov¹, Simeonova A¹, Mihnev N¹*, Simeonov G², Davidov K³ and Kirova G4
¹Clinic of Neurology, Tokuda Hospital Sofia, Bulgaria
²Department of Anesthesiology and Intensive care, Tokuda Hospital Sofia, Bulgaria
³Department of Urology, Tokuda Hospital Sofia, Bulgaria
4Clinic of Imaging Diagnostic, Tokuda Hospital Sofia, Bulgaria
*Corresponding author: Nikolay Mihnev, Clinic of Neurology, Tokuda Hospital Sofia, Bulgaria
Received: June 01, 2015; Accepted: August 05, 2015; Published: August 08, 2015
Abstract
Central pontine myelinolysis (CPM) is heterogeneous group of demyelinating conditions with different etiology, pathophysiological mechanisms, clinical presentation and development. The suspectable main reasons for development of pontine myelinolysis syndrome in patients with alcohol use and malnutrition are basically deficiency of thiamine - vitamin B1 and direct toxic effects of acetaldehyde. Role in cell damage plays changes in serum potassium levels and accompanying these conditions magnesium deficiency.
Early and proper diagnosis of pontine myelinolysis syndrome should be based on acute brain stem dysfunction, history and clinical data of serum electrolyte disturbances, alcohol consumption and malnutrition, as well as typical findings of magnetic resonance imaging (MRI).
We present a case of 42 years old female patient with history of long-term alcohol abuse, normal serum sodium levels and malnutrition after ten-day period of confusion and aggressive behavior develops acute bulbar, pseudo bulbar and quadriparetic syndrome, which requires hospitalization in intensive care unit. Several MRI studies were performed and patient was diagnosed with central pontine myelinolysis syndrome as a result of malnutrition and alcohol abuse. In six months complete reversal of neurological symptoms was observed, but MRI findings persist.
Basic etiological reasons were discussed, as well as possible pathophysiological mechanisms, clinical and diagnostic possibilities for patient with CPM syndrome, systemic alcohol abuse, malnutrition and normal serum sodium levels.
Keywords: Pontine myelinolysis; Malnutrition; Alcohol abuse; Thiamine; Oxidative stress
Background
Central pontine myelinolysis (CPM) is heterogeneous group of demyelinating conditions with different etiology, pathophysiological mechanisms, clinical presentation and development [1,2]. For the first time, the condition was described in 1959 by Adams and colleagues in patients with alcohol consumption and/or malnutrition [1,2]. Later in 1976 Tomlinson describes the case of CPM after rapid correction of low serum sodium levels. Since then, several cases of CPM in patients with alcohol consumption, malnutrition, normal serum sodium level are described [1-3]. In the most cases CPM syndrome is associated with alcohol use and insufficient conditions [3].
In etiological aspect it is a multifaceted disease. Factors which may lead to it are quick correction of low serum sodium levels, chronic alcohol use, insufficient conditions, acute electrolyte imbalance accompanying kidney, liver and gastrointestinal processes, endocrine diseases, late pregnancy toxemia, malignant processes, anorexia and other eating disorders [1-6].
Case Report
We present 42 year female patient hospitalized in a clinic of psychiatry within five days, due to ten-day period of confusion, irrational behavior, anxiety and aggression towards her family. On the fifth day of stay in department of psychiatry due to worsening of her condition she had been hospitalized in intensive care unit of Tokuda Hospital Sofia. The patient was in poor general condition, unable to move, non-contact, with no quantitative changes in consciousness. According to her relatives patient has history for systematically consumed alcohol for years and in recent months patient consumed it in unlimited quantities, refused to eat, had reduction in body weight. Without medical and family history or any data for previous treatment. Without history of fever in recent months or experienced acute viral infections in last year.
Upon admission in intensive care unit, the following changes in somatic status were observed: skin and visible mucosa- pale, expressed ulcers in lips corners. Dry scaly skin on the palms and feet, changes in the nails plate bilaterally. Strongly reduced subcutaneous fat with decreased skin elasticity. Neurological examination upon admission showed mydriasis on the right pupil with missing direct and indirect reaction to light, miotic pupil on the left eye with slow reaction to light, missing bilateral convergence and accommodation reactions, missing smooth tracking eye movements, vertical gaze paresis, spontaneously eyes abduction to the right. Patient was unable to show his tongue upon request, missing bilateral pharyngeal reflexes, absent cough reflex. Quadriparеtic syndrome was observed with right-sided upper central monoplegiya, severe left-sided upper monoparesis, and lower central paraplegia. Muscle tone was decreased in the four limbs. Conjunctival and corneal reflex were reserved. Mandibular reflex was exaggerated. Diminished reflexes for upper limbs, missing knee and Achilles reflexes bilaterally, presented bilateral plantar reflexes, absent cutaneus reflexes. Sustained clonus on the left leg. Sensory function and coordination were not possible to be examined. The patient was in consciousness, without pain response, reserved circadian cycle, catheterized, orotracheal intubated. The following examinations and consultations were performed:
Para clinical test
• laboratory tests data for iron deficiency anemia, low serum levels of vitamin B12, low serum levels of protein, albumin, calcium, potassium, normal serum levels of sodium.
• laboratory tests data for iron deficiency anemia, low serum levels of vitamin B12, low serum levels of protein, albumin, calcium, potassium, normal serum levels of sodium.
• Echocardiography: normal valve apparatus, preserved systolic and diastolic function.
• Electroneurography and Electromyography study showed data for polyneuropathy, affecting sensory and motor nerves, mainly axonal form prevailing in the lower limbs. Myogenic changes mainly in proximal muscle groups.
• Electroencephalography: Generally slow activity without epileptiform changes.
• Magnetic resonance imaging (MRI) of the head upon admission - in the area of the pons is visualized formation with size 22 mm/17 mm., isointense in T1 and hyper intense in T2. After application of gadolinium, contrast enhancement in middle of formation was observed, without perifocal edema/Figure 1,2.
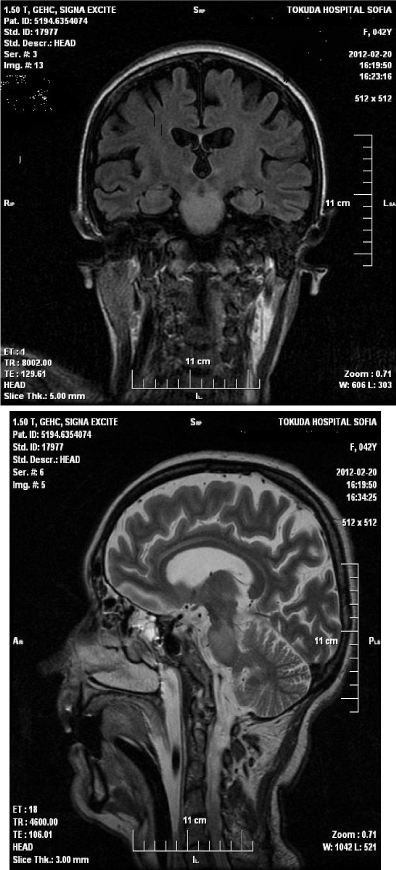
Figure 1: Magnetic resonance imaging (MRI) of the head upon
admission - in the area of the pons is visualized formation with size 22 mm/17
mm., isointense in T1 and hyperintense in T2. After application of gadolinium,
contrast enhancement in middle of formation was observed, without perifocal
edema.
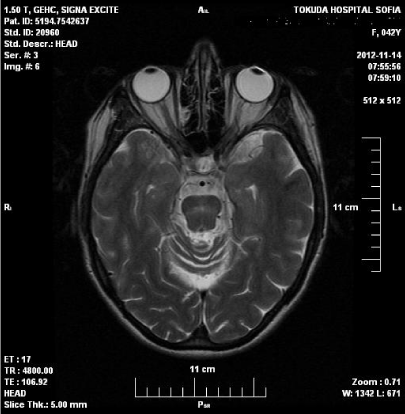
Figure 2: MRI of the head showed reduction of hyperintense zone in the
pons, presence of glial changes, brain atrophy.
• MRI of the cervical segment of the spinal cord with gadolinium enhancement showed no changes.
Consultations with medical specialists
• Cardiologist: no evidence for cardiovascular disease.
• Infectionist - no evidence for inflammatory disease affecting the central nervous system
• Psychiatrist and neuropsychologist- severe alcoholic encephalopathy, in consciousness, do not obey any commands.
• Neurosurgeon: MRI data for tumor formation in the brainstem. Due to the topic of the process patient is not indicated for surgical treatment.
On the second month after hospitalization partial improvement of neurological symptoms was observed: recovered reaction to light, accommodation and convergence bilaterally, vertical and horizontal eye movements. Weakness in four limbs was partially improved, spasticity in the four limbs appeared, and bulbar and pseudo bulbar palsy still persist. The patient was awared and obeyed to elementary commands. Decreased sensation for touch, pain and vibration in lower limbs was observed. Control examinations were performed.
• MRI of the head showed data of hyper intense zone on T2 series and hypo intense on T1 series in the pons. Restriction of diffusion weighted imaging (DWI) series in the pons. Size 23/15 mm. No gadolinium enhancement was observed.
• MRI of cervical, thoracic and lumbosacral segment found degenerative changes, without spinal cord compression.
• Laboratory tests - mild anemia and low serum albumin levels.
Nine months after disease onset significant improvement of neurological symptoms were observed. Polyneuropathy persists with decreased sensation for touch, pain and vibration in lower limbs, missing reflexes in the lower limbs and positive axial phenomena. Laboratory tests were normal. MRI of the head showed reduction of hyperintense zone in the pons, presence of glial changes, brain atrophy./Figure 3.
The following treatment was carried out: intravenous electrolyte infusions, magnesium sulfate, vitamin B1, vitamin B6, vitamin B12, nootropic agents, corticosteroids, rehabilitation.
Discussion
Central pontine myelinolysis is a demyelinating disease, affecting pons, often associated with areas of demyelination in other structures of the central nervous system [1,2] This syndrome mainly divided into two types according to the affected structures: central pontine myelinolysis syndrome and extrapontine myelinolysis syndrome [1]. The importance of osmotic stress in the etiology of CPM gives alternative name- osmotic demyelination syndrome. Etiology and pathogenetic mechanisms are still unclear and discussible [4]. In etiological aspect it is a multifaceted disease [1,3,7-9]. Factors which may lead to it are quick correction of low serum sodium levels, chronic alcohol use, malnutrition conditions, acute electrolyte imbalance, gastroenterological processes, endocrine diseases, immunosuppressive therapy after liver transplantation, late toxemia of pregnancy, anorexia and other eating disorders, low serum potassium levels, inadequate secretion of antidiuretic hormone and malignant formations [1-,7,10].
According Lamp and Yazdi study in 450 cases of pontine myelinolysis, history of alcohol use was found in 39.4 percent of the cases, 21.5 percent were as a result of rapid correction of low serum sodium levels, 17.4 % were after immunosuppressive treatment in patient with liver transplantation [3,4]. Harmful alcohol consumption, electrolyte abnormalities and malnutrition conditions are the most common causes in etiology of CPM [3,9,11,12]. The presence of alcohol consumption was cited as a leading cause in 78% of cases. The basic pathophysiological mechanisms are:
• Osmotic stress as a result of electrolyte abnormalities and changes in extra- and intracellular fluid [9,12,13].
• Changes in peroxidase activity with formation of free radicals leading to cell death [1,9,12,13].
• Changes in formation and function of adenosine triphosphatase (ATP) system in the cells [9,12,13].
Diversity of etiologies and pathophysiological mechanisms determine variations in clinical symptoms of the disease [2,7,14-16]. Severity of the disease varies from asymptomatic finding detected by neuroimaging methods to severe neurological symptoms, coma and death [17-20]. The most common clinical presentation of CPM is combination of psychiatric symptoms- emotional liability, disinhibition, aggression, lack of criticism and neurological symptoms- acute bulbar and pseudo bulbar syndrome, quadriplegic syndrome, changes in consciousness [7,14-16,21]. In some patients only neurological symptoms are observed [14,20,22].
Early and proper diagnosis of CPM should be based on the clinical presentation of acute brain stem dysfunction, history and clinical data for serum electrolyte disturbances, alcohol consumption and malnutrition, and typical MRI findings [1,5,6,11]. CT of the head and stem and evoked potentials may show non-specific changes into the brain stem [23]. The diagnose should be based on MRI data. The earliest changes could be detected on diffusion MRI within 24 hours after initial symptoms [11,19,20,23]. Changes are best seen on T2- weighted images. Sometimes their development can take up to two weeks after the neurological symptoms onset [11,23]. They are hypo intense on T2 and could persist for a long period of time after clinical improvement of the patient [23,24]. In many cases the size of the area did not correlate with the severity of the clinical symptoms. Affected areas are visualized as typical butterfly-like zones of demyelination without inflammation. Absence of inflammation differentiates the syndrome of osmotic demyelination from others demyelinating diseases in which inflammatory component dominates [11,23]. The most common location is central part of the pons and spreading “like a brush fire”, to its base. Rarely can be observed spreading of the process to the midbrain and medulla oblongata [11,20,23,25,26].
The presented case report of young woman with history of longterm alcohol abuse, malnutrition, which after a ten-day period of confusion and aggressive behavior develops acute bulbar, pseudo bulbar and quadriparetic syndrome with characteristic MRI findings, are typical for CPM. As a differential diagnosis tumor process in the pons was considered and rejected by control MRI scans. No medical, family history or other risk factors was revealed, except typical for the disease malnutrition and alcohol abuse. In six months from the beginning of CPM complete reversal of neurological symptoms was observed, but MRI findings still persist.
There are two probable reasons for the development of the CPM syndrome in patients with alcohol use and malnutrition: lack of thiamine (vitamin B1) and direct toxic effects of acetaldehyde. Role in cell injury plays serum potassium levels changes and accompanying these conditions magnesium deficiency [1,2,13,27,28]. Thiamine deficiency as a result of alcohol consumption is associated with insufficient food imports, malabsorption syndrome accompanying alcoholic disease, and impaired use of thiamine in the cells due to transport systems changes necessary for absorption [6,12,27,28]. Thiamine is with exogenous origin, and a cofactor for multiple enzyme systems associated with reuptake of carbohydrate molecules into the cells. The main enzyme systems which are activated by thiamine are: transketolase, pyruvate dehydrogenase (PDH), alphaketoglutarate dehydrogenase (α-KGDH). These circuits are involved in the enzymatic synthesis of nucleic acids, complex sugar molecules, steroids, fatty acids, and for a part of neurotransmitters. They also play role in synthesis of glutathione, which is a part of antioxidant function [12,17,27]. It has been proved their involvement into biochemical cycles- the citric acid cycle and glycolysis, which are associated with processing of glucose molecules. Thiamine featuring in adenosine triphosphate (ATP) synthesis via activation of a series of biochemical chains as a result provides energy for many cellular processes and reactions. Decreased activity of PDH and α - KGDH as a result of thiamine deficiency can lead to decreased ATP synthesis and could cause disability or even death of cell structures. In addition, the proper functioning of the PDH enzyme system is essential for production of neurotransmitter acetylcholine, and for synthesis of myelin, involved in construction of nerves structures myelin sheath [12,13]. The cycle of citric acid and α - KGDH plays role in maintaining glutamate, gamma-amino butyric acid (GABA) and aspartate levels. This is evidence for thiamine participation in cellular metabolism and cellular functioning. Low levels of thiamine, observed in patients with alcohol abuse and malnutrition decreased activity of enzyme systems and lead to changes in cell metabolism leading to cell death [12,27,29]. Leading cause for cells death has osmotic stress as a result of a changes in transport passage trough in and out the cell membrane [12,30]. The process called osmotic demyelinating syndrome leads to release of glutamate, blocking the ion channels and increased intracellular calcium. Other reasons for cells death are changes in peroxidase metabolism, free radicals, and changes in endothelial function and released myelin toxic factor, which leads to disruption integrity of the myelin sheath of the cells [6,12].
Conclusion
Important for diagnose CPM, as it was described in our patient are acute onset brainstem neurological symptoms with a history of psychiatric symptoms, anamnesis for harmful alcohol consumption and malnutrition, electrolyte disturbances, typical MRI findings. Early diagnosis is crucial for good outcome of the disease. Descrypted case report is clinical interest due to the combination of etiological factors, pathophysiological mechanisms, clinical presentation, early diagnostic capabilities and therapeutic response with favorable outcome.
References
- Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto-undescribed disease occurring in alcoholic and malnourished patients. American Medical Association Arch Neurol Psychiatry. 1959; 81: 154–172.
- Adams RD. Living legends: central pontine mielynolisis. Adv Clin Neurosci Psychiatry. 2006; 6: 22-22.
- Bernard M, William O, Michael H. Central pontine myelinolysis. Considerations on etyology, diagnosis and treatment. Neurology. 1979; 29: 21-47.
- Bonham CA, Dominguez EA, Fukui MB, Paterson DL, Pankey GA, Wagener MM, et al. Central nervous system lesions in liver transplant recipients: prospective assessment of indications for biopsy and implications for management.Transplantation. 1998; 66: 1596-1604.
- Charness ME. Brain lesions in alcoholics.Alcohol Clin Exp Res. 1993; 17: 2-11.
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain?J Neuropathol Exp Neurol. 1998; 57: 101-110.
- Ashrafian H, Davey P. A review of the causes of central pontine myelinosis: yet another apoptotic illness?Eur J Neurol. 2001; 8: 103-109.
- Gibson GE, Ksiezak-Reding H, Sheu KF, Mykytyn V, Blass JP. Correlation of enzymatic, metabolic, and behavioral deficits in thiamin deficiency and its reversal.Neurochem Res. 1984; 9: 803-814.
- Laitt RD, Thornton M, Goddard P. Pontine myelinolysis in a normonatraemic alcoholic.Clin Radiol. 1993; 48: 432-433.
- Gocht A, Colmant HJ. Central pontine and extrapontine myelinolysis: a report of 58 cases.Clin Neuropathol. 1987; 6: 262-270.
- Bernsen HJ, Prick MJ. Improvement of central pontine myelinolysis as demonstrated by repeated magnetic resonance imaging in a patient without evidence of hyponatremia.Acta Neurol Belg. 1999; 99: 189-193.
- Hoyumpa AM Jr. Mechanisms of thiamin deficiency in chronic alcoholism.Am J Clin Nutr. 1980; 33: 2750-2761.
- Laforenza U, Patrini C, Gastaldi G, Rindi G. Effects of acute and chronic ethanol administration on thiamine metabolizing enzymes in some brain areas and in other organs of the rat.Alcohol Alcohol. 1990; 25: 591-603.
- Price BH, Mesulam MM. Behavioral manifestations of central pontine myelinolysis.Arch Neurol. 1987; 44: 671-673.
- Razvi SS, Leach JP. Asymptomatic pontine myelinolysis.Eur J Neurol. 2006; 13: 1261-1263.
- Hurley RA, Filley CM, Taber KH. Central pontine myelinolysis: a metabolic disorder of myelin.J Neuropsychiatry Clin Neurosci. 2011; 23: 369-374.
- Martin PJ, Young CA. Central pontine myelinolysis: clinical and MRI correlates.Postgrad Med J. 1995; 71: 430-432.
- Mascalchi M, Cincotta M, Piazzini M. Case report: MRI demonstration of pontine and thalamic myelinolysis in a normonatremic alcoholic.Clin Radiol. 1993; 47: 137-138.
- Miller GM, Baker HL Jr, Okazaki H, Whisnant JP. Central pontine myelinolysis and its imitators: MR findings.Radiology. 1988; 168: 795-802.
- Mochizuki H, Masaki T, Miyakawa T, Nakane J, Yokoyama A, Nakamura Y, et al. Benign type of central pontine myelinolysis in alcoholism--clinical, neuroradiological and electrophysiological findings.J Neurol. 2003; 250: 1077-1083.
- Strub MU, Steck AJ, Fuhr P. Asymptomatic central pontine myelinolysis.Neurology. 1999; 53: 914.
- Norenberg MD, Leslie KO, Robertson AS. Association between rise in serum sodium and central pontine myelinolysis.Ann Neurol. 1982; 11: 128-135.
- Ruzek KA, Campeau NG, Miller GM. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging.AJNR Am J Neuroradiol. 2004; 25: 210-213.
- Sterns RH, Hix JK. Overcorrection of hyponatremia is a medical emergency.Kidney Int. 2009; 76: 587-589.
- Vermetten E, Rutten SJ, Boon PJ, et al. Neuropsychiatric and neuropsychological manifestations of central pontine myelinolysis. Gen Hosp Psychiatry. 1999; 21: 296–302.
- Yuh WT, Simonson TM, D'Alessandro MP, Smith KS, Hunsicker LG. Temporal changes of MR findings in central pontine myelinolysis.AJNR Am J Neuroradiol. 1995; 16: 975-977.
- Juergenson I, Zappini F, Fiaschi A, Tonin P, Bonetti B. Teaching neuroimages: neuroradiologic findings in pontine and extrapontine myelinolysis: clue for the pathogenesis?Neurology. 2012; 78: e1-2.
- Leevy CM, Baker H. Vitamins and alcoholism. Introduction.Am J Clin Nutr. 1968; 21: 1325-1328.
- Laubenberger J, Schneider B, Ansorge O, Götz F, Häussinger D, Volk B, et al. Central pontine myelinolysis: clinical presentation and radiologic findings.Eur Radiol. 1996; 6: 177-183.
- Laureno R, Karp BI. Myelinolysis after correction of hyponatremia.Ann Intern Med. 1997; 126: 57-62.