
Case Report
Austin J Clin Neurol 2015; 2(9): 1078.
Different Phenotypes at Possibly Shared Genotype of Migraine Family
Yalın OÖ¹*, Edgünlü TG² and Özge A³
¹Department of Neurology, Istanbul Education and Research Hospital, Turkey
²Department of Medical Biology and Genetics, Mugla School of Health Sciences, Turkey
³Department of Neurology, Mersin University School of Medicine, Turkey
*Corresponding author: Osman Özgür Yalın, Department of Neurology, Istanbul Education and Research Hospital, Istanbul, Kasapİlyas Caddesi Org, Abdurrahman, Nafiz Gürman Cd. No: 18 Fatih/Istanbul, Turkey
Received: June 12, 2015; Accepted: August 10, 2015; Published: August 12, 2015
Abstract
In this paper we described clinical features of a family including mother and all three children with migraine. Migraine is regarded as a chronic disorder with episodic manifestations and also is progressive for at least some of the patients. Our nuclear family consist mother and her all-three children with migraine. We pointed clinical differences and comorbidity of migraine with aura (MwA) and migraine without aura (MwoA) and existence of cutaneous allodynia (CA) at this family. We believe in that family or twin studies could help us not only to reveal pathophysiology of migraine but also could contribute us to understand possible effects of clinical variables (such as aura, cutaneous allodynia).
Keywords: Migraine; Inheritance; Aura; Cutaneous allodynia
Introduction
Migraine is a common neurovascular disorder characterized by disabling headache attacks and associated with accompanying symptoms. Migraine affects more than 10 % of the population and is one of the major causes of health costs for all over the world [1]. Genetic predisposition of migraine is obvious and family history is present more than half of the patients. However responsible genes or inheritance form are still uncovered excluding hemiplegic migraine. To this date, migraine investigations have advanced current knowledge about genetic contributions and the pathophysiology of disease. Generally migraine genetic basis depends on polygenic and multifactorial. Familial hemiplegic migraine (FHM) is a rare monogenic subtype of migraine with motor aura symptoms. On the other hand many candidate genes analyzed for common migraine types (including 5-HT related genes, dopamine-related genes, and glutamate receptors). Vascular (ACE, MTHFR, NOS2, NOS3, NOTCH3), hormonal (ESR-1, ESR2, CYP19A1) and inflammatory candidate genes (TNFA, TNFB, COX2) are also investigated for associations of migraine [2-8]. International Headache Genetics Consortium (IHGC) analyzed the first Genome wide association studies (GWAS) of migraine in 2010. Genetic markers could explain phenotypic variation in many common forms of migraine [9].
Migraine phenotype could have different presentations and about a third of patients with migraine have attacks with aura (Russell 1995) [10]. Aura is defined as clinical transient disturbances attributed to the brain dysfunction [11]. The most common form of the aura is visual aura, sensory and aphasic aura expresses less commonly. Russel & Olesen precisely described migraine aura spectrum in their paper. Visual symptoms were most frequent (99%), followed by sensory (31%), aphasic (18%) and motor (6%) symptoms [12].
Migraine is associated with an increased risk of cardio- and cerebrovascular disease, and this relationship is confirmed by large prospective studies [13]. Recently according to a systematic review elevated risk is reported particularly in the case of aura and female sex and denoting doubts of benignity of migraine [14]. Currently there is limited data reveals natural course of MwA and there is necessity of long-term follow-up studies to reveal enigma of this subjects [15].
In this report we aimed to discuss migraine phenotypic variability in the light of a nuclear family.
Patient Descriptions
Mother; GB, 53 years old: She remembers her first headache at 15 years old, before 38 years ago. Some of her headache attacks include aura, containing visual and sensorial perceptions. Her typical attack starts by scintillating scotoma, blurring at left visual field and 5-10 minutes later headache begins. After headache started almost every time spontaneous sensorial symptoms occurs at right hand and leg. Paresthesia starts distal parts of extremities and gradually spreads proximal and face region and lasts about 30 minutes. After aura phase completed and ended, headache gradually increases its severity and lasts about two days. She describes 4 attacks per month for one year and states attack numbers reduced since menopause without phenotypic evolution. She described prominent CA symptoms and her Allodynia Symptom Checklist (ASC-12) score was twelve. She has history of hypertension, diabetes mellitus, and coronary artery disease.
First male child; IBM, 25 years old male: He have headache for 5 years. He describes visual auras, with scintillating scotoma for 10 minutes at some of attacks. Headache attacks gradually increases its severity and lasts approximately 8 hours. He describes 5 attacks per month. He does not have CA, and ASC-12 score was zero. He has history of childhood epilepsy that resolved completely. He is not using any medication regularly.
Secondmale child; ASM, 21 years old male: He has headache for 10 years. We asked carefully but he did not notice any kind of aura. Headache attacks gradually increases its severity and lasts approximately 12 hours. He describes 10 headache days per month. He has prominent allodynia symptoms. He has history of hypertension, hyperlipidemia, and coronary artery disease. He doesn’t using any medication regularly and smokes cigarette 1 pack/day for 10 years.
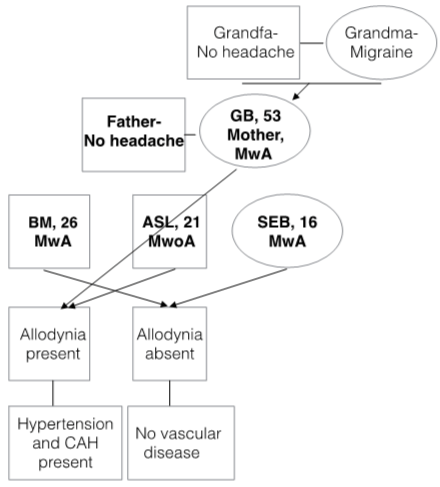
Figure 1: Family Pedigree of migraine and comorbidity.
Female child: SEB 16 years old female. She remembers her first attack at 12 years old, for four years. She described typical visual aura preceding to headache, including scintillating scotoma, visual field defects lasts for 5-20 minutes. Within headache phase, aura symptoms resolve for 5 minutes. Headache gradually increases severity and lasts about two days. She describes 10 headache attacks per month for 1 year. She doesn’t have any chronic illness history, and not smoking. Detailed features of patients explained at Table 1.
GB (mother)
İBM (boy)
ASM (boy)
SEB (girl)
Age
53
25
21
16
Education/years
15
8
15
11
Headache onset/years
38
5
10
4
Freequency
4
4
10
10
Duration (hours)
72
8
12
48
Pain Intensity (VAS)
9
8
10
8
Throbbing headache
Present
Present
Present
Present
Associationof physicalactivity
Present
Absent
Present
Present
Unilateral headache
Present
Absent
Present
Present
Accompanying features
Nausea
Present
Present
Present
Present
Vomting
Present
Present
Absent
Absent
Phonophobia
Present
Present
Absent
Absent
Photophobia
Present
Present
Present
Present
Allodynia
Present
Absent
Present
Absent
Blood pressure
120/86
140/80
150/92
118/66
Body mass index
26,7
29,4
33,0
22,1
Neurological exam.
Normal
Normal
Normal
Normal
Comorbidities
DM, HT, CAH
Childh. epil
HT, HL, CAH
Absent
Meication over use
Absent
Absent
Absent
Absent
Triggers
Mens.,Skipping meals, Stress
Mens.,Skipping meals, Stress
Mens.,Skipping meals, Stress
Mens.
Sensoriel Aura
Present
Absent
Absent
Absent
Visual Aura
Present
Present
Absent
Present
ASC-12 Score*
12
2
8
0
MRI
PV leukaryosis
-
-
N
Final Diagnosis (IHC-3 Edition)
MwA
MwA
MwoA
MwA
*ASC-12 (Allodynia Symptom Checklist-12) (cut off value regarded as >4 points) (Lipton 2008), CAD (coronary artery disease), Child. epil.: Childhood epilepsy, DM: diabetes mellitus, HT: hypertension, HL (hyperlipidemia), Mens: Menstruation, MwA: migraine with aura, MwoA: migraine without aura. MRI: magnetic resonance imaging,
Table 1: Clinical features of patients.
Discussion
This nuclear family includes mother and her three children and all of them have migraine. Mother states her mother and grandmother have also migraine with very severe attacks and her father, grandfather and her husband don’t have headaches. It seems same inherited form of disease but disease phenotype is different for all patients including MwA and MwoA. Recently genetic researches showed various migraine related genes including ion channels and transporters, abnormal neurotransmission and vascular dysfunction related genes. The GWAS methodology made possible genetic variants in neuronal and vascular pathway. Another suggestion is epigenetic effect on migraine. There is also evidence suggests epigenetics could have role in migraine pathophysiology [16,17]. As a polygenic migraine some researchers have theorized migraine could be a tradeoff and exists as a spectrum of susceptibility. Majority of the population is falling in the ‘heterozygous’ zone between never experiencing headache and frequent, incapacitating headache.
Aura and cardio, cerebrovascular risk of migraine
Mother and her small boy (ASL) have comorbid vascular risk factors but they do not share same migraine subtype (mother have MwA with visual and sensorial symptoms, but the son (ASL) didn’t report any aura symptom) (Table 1). This could not be explained by disease evolution at this family because mother remembers auras at her young ages and also youngest girl have visual auras since 14 years old. MwA have important differences from MwoA, especially for its attribution of increased cardio- and cerebrovascular disease risk. In a large, prospective cohort of women, active MwA was associated with increased risk of major CVD, myocardial infarction, ischemic stroke, and death due to ischemic CVD, as well as with coronary revascularization and angina. They also report MwoA was not associated with increased risk of any cerebrovascular event [13]. Similarly Gudmundsson et al, in a population based cohort study reported an increased risk of cardiovascular disease, coronary heart disease and stroke in patients with migraine with aura but not for migraine without aura [18]. Bigal et al. identified that all types of migraine headache were associated with an increased risk of stroke, but they also report highest risk among migraine with aura patients [19]. Scher et al reports that MA was associated with a significantly increased risk for hyperlipidemia, hypertension, and elevated Framingham scores at their population based study [20]. Moreover they report a polymorphism in the methyltetrahydofolate reductase gene (T677T) is associated with moderately elevated levels of homocysteine, which is associated with cerebrovascular disease [20,21]. Recently Sacco & Kurth in a meta-analysis reports that the majority of available data have indicated migraine with aura can be considered as an independent risk factor for ischemic stroke [22]. Soon there is a distinct difference between MwA and MwoA as an independent vascular risk factor. As a sum of knowledge it can be proposed that MwA could not be regarded only a benign epiphenomenon as a primary headache disorder, but also could be assumed an independent cardio -cerebrovascular risk factor. Herein there is a distinct difference between MwA and MwoA as an independent risk factor. We could debate some possibilities of this difference at our family. Firstly some environmental factors could have effects for aura presentation. Secondly in this family migraine could be evolving with disease duration and aura could be added to picture of disease some time. MwA and MwoA and relationship of vascular risk factor could not be explained at this case report and this is not the scope of this report. But we wanted to point that family or twin follow-up studies could help us to solve this enigma.
Cutaneous allodynia and comorbidity
Although mother and second boy ASL don’t share aura, they have some different features from other family members. Both of them report significant cutaneous allodynia (CA) for a long time and also have vascular comorbidity (Table 1). CA is characterized by pain provoked by stimulation of the skin that would ordinarily not produce pain [23]. CA is considered to be a clinical expression of sensitization of the nociceptive neurons in the trigeminal nucleus caudalis, which receives inputs from the dura mater and periorbital skin [24]. Epidemiological studies suggest that approximately two thirds of migraineurs experience allodynia during a migraine attack [23]. There is evidence suggesting that the migraine patients are mostly non-allodynic during the first years of disease and eventually develop allodynia [24,25]. And it is supposed that repeated migraine attacks over the years have cumulative structural effects on trigeminovascular pathway [26]. And this theory is supported by evidence of iron deposition at periaqueductal gray matter (PAG) in patients with a long history of migraine [27]. In a large population based study supposed that CA could be a representative of the repeated attacks of migraine lead to the development of allodynia. On the other hand, they notice that allodynia may be a marker of risk for frequent and severe attacks [23]. They also report the odds of severe CA increased 3, 5 in MwA. CA existence could be explained by disease duration in our family, CA present mother and son’s disease duration are significantly longer than other family members (Table 1). But also they have comorbid vascular risk factor diseases (DM, HT, HL, CAH) differently from other family members. This report could not reveal this relationship. However it should be keep in mind that CA may be a marker of risk of comorbid disease. Evaluation of this hypothesis at large population based studies could help us to reveal exact significance of CA at migraine population.
Conclusion
Although there is growing evidence about migraine pathophysiology and disease course. There are also many aspects of migraine remaining to be enlightened. We believe in that long-term follow-up studies could help us to understand disease evolution and prognosis. Current knowledge is supporting that migraine could be defined as an independent risk factor for cardio; -cerebrovascular diseases and patient should be explored about additional risk factors.
References
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007; 68: 343-349.
- Riant F, Ducros A, Ploton C, Barbance C, Depienne C, Tournier-Lasserve E. De novo mutations in ATP1A2 and CACNA1A are frequent in early-onset sporadic hemiplegic migraine. Neurology. 2010; 75: 967-972.
- Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996; 87: 543-552.
- Ghosh J, Joshi G, Pradhan S, Mittal B. Potential role of aromatase over estrogen receptor gene polymorphisms in migraine susceptibility: a case control study from North India. PLoS One. 2012; 7: e34828.
- Yilmaz IA, Ozge A, Erdal ME, Edgünlü TG, Cakmak SE, Yalin OO. Cytokine polymorphism in patients with migraine: some suggestive clues of migraine and inflammation. Pain Med. 2010; 11: 492-497.
- Rainero I, Grimaldi LM, Salani G, Valfrè W, Rivoiro C, Savi L, et al. Association between the tumor necrosis factor-alpha -308 G/A gene polymorphism and migraine. Neurology. 2004; 62: 141-143.
- Corominas R, Sobrido M J, Ribases M, Cuenca-León E, Blanco-Arias P, Narberhaus B, et al. Association study of the serotoninergic system in migraine in the Spanish population. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2010; 153: 177-184.
- Todt U, Netzer C, Toliat M, Heinze A, Goebel I, Nürnberg P, et al. New genetic evidence for involvement of the dopamine system in migraine with aura. Hum Genet. 2009; 125: 265-279.
- Fan X, Wang J, Fan W, Chen L, Gui B, Tan G, et al. Replication of migraine GWAS susceptibility loci in Chinese Han population. Headache. 2014; 54: 709-715.
- Russell MB, Rasmussen BK, Thorvaldsen P, Olesen J. Prevalence and sex-ratio of the subtypes of migraine. Int J Epidemiol. 1995; 24: 612-618.
- Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002; 346: 257-270.
- Russell MB1, Olesen J . A nosographic analysis of the migraine aura in a general population. Brain. 1996; 119 : 355-361.
- Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006; 296: 283-291.
- Tana C, Tafuri E, Tana M, Martelletti P, Negro A, Affaitati G, et al. New insights into the cardiovascular risk of migraine and the role of white matter hyperintensities: is gold all that glitters? J Headache Pain. 2013; 14: 9.
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013; 33: 629-808.
- Passaro D, Rana G, Piscopo M, Viggiano E, De Luca B, Fucci L. Epigenetic chromatin modifications in the cortical spreading depression. Brain Res. 2010; 1329: 1-9.
- Eising E, A Datson N, van den Maagdenberg AM, Ferrari MD. Epigenetic mechanisms in migraine: a promising avenue? BMC Med. 2013; 11: 26.
- Gudmundsson LS, Scher AI, Aspelund T, Eliasson JH, Johannsson M, Thorgeirsson G, et al. Migraine with aura and risk of cardiovascular and all cause mortality in men and women: prospective cohort study. BMJ. 2010; 341: c3966.
- Bigal ME, Kurth T, Santanello N, Buse D, Golden W, Robbins M, et al. Migraine and cardiovascular disease: a population-based study. Neurology. 2010; 74: 628-635.
- Scher AI, Terwindt GM, Picavet HS, Verschuren WM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology. 2005; 64: 614-620.
- Scher AI, Terwindt GM, Verschuren WM, Kruit MC, Blom HJ, Kowa H, et al. Migraine and MTHFR C677T genotype in a population-based sample. Ann Neurol. 2006; 59: 372-375.
- Sacco S, Kurth T. Migraine and the risk for stroke and cardiovascular disease. Curr Cardiol Rep. 2014; 16: 524.
- Lipton RB, Bigal ME, Ashina S, Burstein R, Silberstein S, Reed ML, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008; 63: 148-158.
- Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000; 47: 614-624.
- Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004; 55: 27-36.
- Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol. 2012; 8: 89-99.
- Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001; 41: 629-637.