
Research Article
Austin J Clin Neurol 2015; 2(10): 1083.
Low Serum Levels of Antiepileptic Drugs in Patients with Epilepsy-Bad Compliance or Something Else?
Rasheva M¹, Staikov I¹*, Svinarov D², Mihnev N¹, Neykov N¹ and Staneva M³
¹Department of Neurology and Sleep Medicine, Tokuda Hospital, Bulgaria
²Central Laboratory of TDM & Clinical Pharmacology, Medical University, Bulgaria
³Department of Vascular Surgery and Angiology, Tokuda Hospital, Bulgaria
*Corresponding author: Ivan Staikov, Department of Neurology and Sleep Medicine, Tokuda Hospital, 51 B Nikola Vaptzarov blvd., 1407 Sofia, Bulgaria
Received: August 11, 2015; Accepted: October 20, 2015; Published: October 22, 2015
Abstract
Background: About 30% of all patients with epilepsy are estimated to have drug-resistant epilepsy. Literature data shows poor subject compliance and low serum levels of antiepileptic drugs (AEDs) in 20-50% of patients with epilepsy.
Objective: To establish the frequency and causes of low sub-therapeutic serum levels of AEDs in epilepsy patients with inadequate seizures control.
Methods: The study included 61 patients with epilepsy, 29 men and 32 women aged between18 to 80 (average age of 42.2). All patients are with active epilepsy, in which epileptic seizures continue and/or electroencephalography shows paroxysmal activity.
Results: Correlation between low serum levels of AEDs and age was found. Low serum levels were observed more frequently in younger age groups compared with older age groups. Patients with idiopathic generalized epilepsy (IGE) more often have sub-therapeutic plasma levels of AEDs compared with those with partial epilepsy - P <0,02. Serum levels are lower in patients with frequent seizures (over 2/monthly) compared to those with a rare seizures (less than 2/monthly) - P <0.02.
Conclusion: In conclusion, the results from our study show that the administered doses of AEDs in monotherapy or in combination of two or more AEDs are low at the most cases. In 1/5 of the cases there is bad compliance. Low serum levels of AEDs are more frequent in younger patients, those with IGE and in patients with more frequent seizures.
Serum levels of the AED in patients with unsatisfied control of epileptic seizures should be examined prior to diagnosis “drug resistant epilepsy”.
Keywords: Epilepsy; Antiepileptic drugs; Seizures
Background
About 30% of all patients with epilepsy are estimated to have drug-resistant epilepsy [1,2]. The new International League Against Epilepsy (ILAE) consensus definition of drug-resistant epilepsy requires “failure of two tolerated, appropriately chosen and used antiepileptic drug schedules to achieve sustained seizure freedom” (i.e., either 12 months or three times the longest seizures free period) [2]. Adequate choice is based on knowledge of the physician - some antiepileptic drugs (AEDs) are inappropriate in some epileptic syndromes and may aggravate them [3]. Adequate administration means dosage corresponding to the weight, age, sex and metabolism. Right treatment depends on cooperation (subject compliance) of the patient. Literature data shows poor subject compliance and low serum levels of AEDs in 20-50% of patients with epilepsy [4]. Patients with drug resistant epilepsy use rational polytherapy with a combination of AEDs with different mechanisms of action. In the case of polytherapy pharmacokinetic interactions are possible [1,5,6]. It was found that 28% of seizures in patients with epilepsy are as a result of low serum levels of AEDs [7,8,9].
Objective
To establish the frequency and causes of low sub-therapeutic serum levels of AEDs in epilepsy patients with inadequate seizures control.
Methods
The study included 61 patients with epilepsy, 29 men and 32 women aged between18 to 80, the average age of 42.2, the standard deviation - 20,3. All patients are with active epilepsy [10], in which epileptic seizures continue and/or electroencephalography (EEG) shows paroxysmal activity (Table 1).
Patients were followed up every six months visits including medical history, neurological examination, EEG, AEDs serum level determination. For subject compliance assessment indirect methods were used, such as an interview with the patient or patient’s caregiver, and direct method by calculating the variability of serum levels in serial measurement. Compliance is good, when variation in serum levels of AED is below 20% [4]. Some patients were treated with low doses of AEDs for their weight, but compliance was good. Low dose treatment is defined as dose with >25% below the lower limit for each AED [1]. Serum levels of valproate (VPA), carbamazepine (CBZ), lamotrigine (LTG), oxcarbazepin (OXC) and its active metabolite (MHD), topiramate (TPM) were examined and in some patients – serum levels of levetiracetam (LEV) - mmol/l.
Results and Discussion
The most commonly used AEDs in monotherapy or in combination are CBZ and VPA (Table 2), followed by LEV and LTG, which is consistent with findings from other European studies [11]. Despite the advantages of mono therapy, about 50% of epilepsy patients are treated with combination of 2 AEDs, 13% - 3-AEDs because of lack of efficiency [12].
In 28 (45.9%) patients sub-therapeutic serum levels of the AEDs were established. Only 12 (20%) confessed that they take lower dose or did not take regular AEDs. In 11 patients data were confirmed by the variability in the levels of AEDs over 20%. Our results are similar to literature data [8], although the percentage of poor compliance in patients with epilepsy varies enormously and reached 70-80% in some studies [13].
Prescribed dosages of AEDs also vary widely, and often lower when calculated per kg body weight (in 10 patients). Most often refers to CBZ (5 patients taking 150-300 mg/day in weight over 78 kg and 200-400 mg/day in weight 82 and 88 kg). Usually gabapentin (GP) and pregabalin (PGB) are administered at low doses, but probable cause is that they are often used as аdditional therapy, in older patients, in which metabolism is generally slower.
Correlation between low serum levels of AEDs and age was found. Low serum levels were observed more frequently in younger age groups compared with older age groups - P = 0.43 (Figure 1).
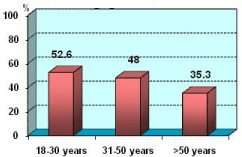
Figure 1: Low serum levels of AEDs according age n=61.
Serum levels of AEDs in women tend to be lower than men, but differences are not statistically significant (Figure 2).
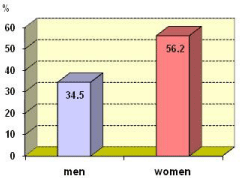
Figure 2: Subtherapeutic serum levels according gender n=61.
Patients with idiopathic generalized epilepsy (IGE) more often have sub-therapeutic plasma levels of AEDs compared with those with partial epilepsy - P <0.02 (Figure 3).
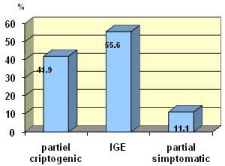
Figure 3: Low serum level of AEDs according epilepsy etiology n=61.
Serum levels are lower in patients with frequent seizures (over 2/monthly) compared to those with a rare seizures (less than 2/ monthly) - P <0.02 (Figure 4). In patients with very rare seizures (1 in 6 or more months) the results are intermediate.
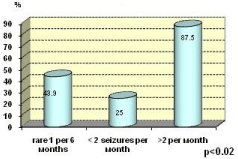
Figure 4: Low serum level and frequency of the seizures per month.
According some authors two AEDs in lower doses has better effect over seizures than high doses of one AED [13]. This conception is clinical behavior of many neurologists. In these cases, the risk of drug interactions is high. There are two types of interactions: pharmacodynamic- do not cause changes in the serum levels of AEDs and pharmacokinetics, with the change in concentration of at least one of the medications [1,5,7,13-15].
The most important pharmacokinetic interactions are those that involve the cytochrome P450 enzymes of hepatic metabolism [16]. From older generation AEDs these are CBZ, phenytoin (PHT), phenobarbital (PB) and primidone. They induce the activity of cytochrome P450 (CYP) enzymes, glucuronate transferase (GT) and epoxide hydrolases and cause reduction of serum concentrations of concomitant drugs [16]. Conversely, VPA causes clinically significant interactions by inhibiting the metabolism of some AEDs, the most significant of PB and LTG [17].
In our study, in 9 of 10 patients (90%) treated with CBZ, serum levels of the second AED are low (in 3/3 - of VPA, 3/3 - of the TPM, 2/3 - of the LTG, 1/2 - of TPM + LEV). It was found that CBZ significantly reduces concentrations of TPM, VPA and LTG [17]. Similar results were seen in combination with other AEDs - metabolic inducers (phenytoin -PHT and PB). The level of CBZ is also low in 5 (50%) - 2/3 as an additional therapy with LTG, 2/3 with VPA and 1/4 - with the TPM. CBZ was prescribed in low dosage in 2 patients and compliance was bad in 3 patients. The metabolism of CBZ could be auto-inducted, also induced by barbiturates and PHT [17].
In 5 patients treated with OXC serum levels of the drug and MHD are in therapeutic range, and the concomitant drug serum levels (GP, PGB, LEV, LEV + LTG) has not been evaluated.
From 13 patients on combination therapy with valproate in 4 (30.8%) - serum levels of second drug is low. VPA was in subtherapeutic levels in 5 patients (38.5%) - in 2 with CBZ, in 2 - with LTG and in 1 - with LEV. Although VPA is enzyme inhibitor, often causes reduction in serum levels of CBZ and other medications when it is used in combination [18]. VPA addition to PHT causes displacement of phenytoin from the plasma proteins with connection of VPA, reduction of total concentration of the PHT, although the concentration of the free, pharmacologically active portion of the PHT is not changed. Even slight increase of PHT dose, can lead to toxicity [16]. In patients treated with CBZ, add-on therapy with VPA may increase the concentration of the active metabolite CBZ-10,11- epoxide by inhibition of epixid hydrolase without changes in the concentrations of AEDs [16]. The most important of inhibitory effects of VPA are inhibition of LTG and PB metabolism with increasing the levels of these AEDs by about 50% [19].
In polytherapy with LTG in 5/9 patients’ serum level of second AED is low. Serum levels of LTG is low at 3/9 - 2 when combined with CBZ and 1 - with PHT. Results from several studies suggest that serum concentrations of VPA, PHT, CBZ, PB does not change when added to LTG [20].
From 13 patients on polytherapy with LEV and other AED, in 5 (36.5%) serum levels of concomitant AEDs are below the therapeutic range (2 on VPA, 2 on LTG, 1 on CBZ). Serum levels of LEV are evaluated only in 5 patients and were within normal range. LEV is not an inducer or inhibitor of liver enzymes and has no clinically significant interactions [16,20].
From 5 patients on combined therapy with TPM in 3 of them the serum levels of the concomitant drug are subtherapeutic-2-of CBZ and 1-of VPA. TPM levels are very low (>50%) in combination with CBZ and PHT. The most authors highlight the low interactions potential of TPM with others AEDs as it is not an inducer or inhibitor of cytochrome P450 enzymes, when administered at therapeutic doses. But his metabolism is subject to induction by enzyme-inducing AEDs [19].
The results from this study confirm the benefits in pharmacokinetics of newer AED-GP, LTG, LEV, TPM. These AEDs are not enzyme inducers or inhibitors [16], but some of them may be affected by metabolically mediated drug interactions [17]. No clinically significant drug interactions for LEV, GP, PGB, were observed [5,12]. LTG and TPM are the most sensitive to interactions. TPM metabolism is more susceptible to induction, than inhibition [5].
Conclusion
In conclusion, the results from our study show that the administered doses of AEDs in monotherapy or in combination of two or more AEDs are low at the most cases. In 1/5 of the cases there is bad compliance. Low serum levels of AEDs are more frequent in younger patients, those with IGE and in patients with more frequent seizures.
Serum levels of the AED in patients with unsatisfied control of epileptic seizures should be examined prior to diagnosis “drug resistant epilepsy”.
References
- Brodie MJ. Management Strategies for refractory Localization-Related Seizures. Epilepsia. 2001; 12: 27-30.
- Kwan P, Brodie MJ. Definition of refractory epilepsy:defining the indefinable? The Lancet Neurol. 2010; 9: 27-29.
- Thomas P, Genton P, Gelisse P, Wolf P. Epilepsie mioclonique juvenile. In: Les syndromes epileptiques de l`enfant et de l`adolescent. 4th edtn. Roger J, Bureau M, Dravet CH, Genton P, Tassinari CA, Wolf P, editors. John Libbey Eurotext Ltd. 2005; 367-388.
- Leppik IE. Compliance in the treatment of epilepsy. In: The treatment of epilepsy. 2nd edtn. Willie E, editor. Williams and Wilkins. 1997; 779-784.
- Johannessen C, Patsalos Ph N. Drug interactions involving the new secondand third-generation antiepileptic drugs. Expert Rev Neurother. 2010; 27: 119-140.
- St Louis EK. Truly rational polytherapy: maximizing efficacy and minimizing drug interactions, drug load, and adverse effects. Curr Neuropharmacol. 2009; 7: 96-105.
- Cramer JA, Mattson RH. Compliance with AED therapy. In: Antiepileptic drugs. 4th edtn. Levy RH, Mattson RH, Meldrum BS, editors. Raven Press, Ltd, New York. 1995; 15: 149-159.
- Mattson RH, Cramer JA, Collins JF and VA Epilepsy cooperative study group. Aspects of compliance in epilepsy: taking drugs and keeping clinic appointments. In: Schmidt D, Lepik IE, editors. Compliance in epilepsy. Amsterdam. Elsevier. 1988; 26: 111-118.
- Stanaway L, Lambie DG, Johnson RH. Noncompliance with anticonvulsant therapy - a cause for seizures. N Z Med J. 1985; 98: 150-152.
- Beghi E. Epilepsy. Curr Opin Neurol. 2007; 20: 169-174.
- Pickerell WO, Lacey AS, Thomas RH, Lyons RA, Smith Ph EM, Rees MI. Trends in the first antiepileptic drug prescribed for epilepsy between 2000 and 2010. Seizure. 2014; 23: 77-80.
- Sander JW. The use of antiepileptic drugs – principles and practice. Epilepsia. 2004; 45: 28-34.
- Deckers CLP, Hekster JA, Keyser A, van Lier HJJ, Meinardi H, Renier WO. Monotherapy versus polytherapy for epilepsy: a multi-center double-blind randomized study. Epilepsia. 2001; 42: 1387-1394.
- Besag FMC, Berry D. Interactions between antiepileptic and antipsychotic drugs. Drug Safety. 2006; 29: 95-118.
- EURAP Study group. Seizure control and treatment in pregnancy. Observations from the EURAP Epilepsy Pregnancy Regisrtry. Neurology. 2006; 66: 354-360.
- Patsalos Ph N, Perucca E. Clinically important drug interactions in epilepsy: general features and interactions between antiepileptic drugs. Lancet Neurology. 2003; 2: 347-356.
- Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2005; 61: 246-255.
- Sun MZ, Deckers CLP, Liu YX, Wang W. Comparison of add-on valproate and primidone in carbamazepine-unresponsive patients with partial epilepsy. Seizure. 2009; 18: 90-93.
- Lyseng-Williamson KA, Yang LPH. Topiramate. A review of its use in the treatment of epilepsy. Drugs. 2007; 67: 2231-2256.
- Benedetti MS. Enzyme induction and inhibition by new antiepileptic drugs: a review of human studies. Fundam Clin Pharmacol. 2000; 14: 301-319.