
Case Report
Austin J Clin Neurol 2017; 4(3): 1109.
Demyelinating Neuropathy of the 1a Afferent Nerve Fibers
Partanen J1* and Auranen M2
1Department of Clinical Neurophysiology, University of Helsinki, Finland
2Department of Neurology, University of Helsinki, Finland
*Corresponding author: Juhani Partanen, Department of Clinical Neurophysiology, University of Helsinki, Jorvi Hospital, P.O. Box 80000029 HUS, Helsinki, Finland
Received: April 12, 2017; Accepted: May 19, 2017; Published: May 29, 2017
Abstract
We describe a female patient with a selective demyelinating disease of the 1a afferent sensory nerve fibers. After suffering from fever for 3 days the patient developed disorder of balance, dizziness, muscle cramps and altered sense of position and muscle tension of the trunk and extremities. Neurological examination revealed positive Romberg´s sign with no improvement during follow-up of 8 years. Laboratory and imaging studies showed no remarkable findings. However, ENMG demonstrated a permanent slow conduction affecting selectively the 1a afferent nerve fibers. We conclude that the patient suffered from an acute and probably immune-mediated demyelinating disease restricted to 1a-afferent nerve fibers, with a prompt remission but persistent sequelae.
Keywords: Dysimmune neuropathy; Guillain-Barré syndrome; Nerve conduction velocity; 1a afferents; Muscle spindle; Muscle cramps
Introduction
Polyneuropathy means dysfunction of the peripheral nerves. There are several types of polyneuropathies: axonal, demyelinating [1] and those associated with metabolic changes [2]. Polyneuropathy may selectively affect either heavily myelinated Aα and Aβ nerve fibers or thin Aδ and C nerve fibers [3], or be restricted to either motor or sensory nerve fibers [4]. It is thus essential to investigate the possible alteration in every nerve fiber group (Figure 1). We describe a patient who suffered from a selective demyelination of 1a-afferent Aα nerve fibers.
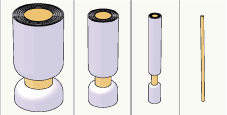
Figure 1: The different classes of the sensory nerve fibers: Aα (diameter
13-20 μm), Aβ (6-12 μm), Aδ (1-5 μm) and C (0.2-1.5 μm) [14]. The Aα fibers
represent mainly the proprioceptive afferent receptors of the muscle spindles
and the Aβ fibers represent the mechanoreceptors of the skin. The Aδ and
C nerve fibers represent the III and IV afferents, for example in the muscle.
Case Presentation
A 55-year old female patient had a three-day fever in July 2008. Fever was associated with difficulty breathing but without any cough or rhinitis. In about a month persistent neurological symptoms developed, consisting of difficulty with balance, dizziness and muscle spasms, as well as strange dysesthesias: feeling of hard muscle tension and feeling that the trunk or the extremities get into distorted positions. An essential finding was also pathological Romberg´s sign, with eyes shut the patient fell backwards but with eyes open she could stand on one leg. The first ENMG study [5] was performed 2.5 years after the appearance of neurological symptoms and a demyelinating neuropathy of the 1a (Aα) nerve fibers [6] was discovered (Figure 2,3) which persisted in the follow-up (Table 1).
8/2008
1/2009
8/2010
2/2011
8/2011
10/2012
1/2013
8/2016
Disorders of the balance, dizziness, tinnitus, difficulty concentrating and sleep.
Weber: lateralization to the right, Romberg´s sign pathological.
Perfusion and MRI of the brain normal. Liquor leucocytes 6 E6/l (normal ad 3E6/l), Erythrocytes 4 E6/l (ad 0 E6/l), Li-prot 568 mg(l (150-450 mg/l). Li-glucose, IgG index, Borrelia antibodies, tick encephalitis antibodies, mycoplasma pneumonia antibodies and TPHA were normal. Blood picture, Mg, Ca, Pi, liver- and kidney studies, B12, thyreoidea studies, and borrelia antibodies were normal.Disorders of balance, dizziness, sleep disturbances. Neuro-otological studies and EEG normal.
Disorders of balance, dizziness, cramps, dysesthesias, muscular fatiguability .
Romberg´s sign pathological. MRI of the brain and spinal cord normal. Neuro-ophtalmological study normal. Creatine kinase, aldolase, lactate normal.ENMG: 1a afferent slowing, all other neurography normal, needle EMG of the extremities and trunk normal. Blood sedimentation rate, complement C3 and C4, immunoglobulins, serum protein electrophoresis, studies for rheumatic disease normal. 2-h value in glucose tolerance test low (1.9-2.5 mmol/l), fasting glucose normal, S-insuline normal, fS-C-Pept, P-Pi, S-Korsol, and carnitinemetab. normal.
Muscle cramps spreading during water running, from the foot muscles to the more proximal lower extremity muscles. ENMG (Table 2).
Disorders of the balance, muscle cramps, restriction of the weekly working hours, ENMG. Molecular genetic study of dystrophia myotonica type 2 normal.
Symptoms and signs as previously. Muscle ion channel study [11] normal. Spiroergometry and O2-consuption normal.
Symptoms and signs continuous. ENMG, SEP.
Table 1: Neurological symptoms and signs and investigations of the patient.
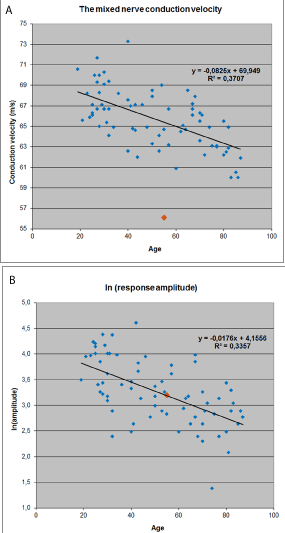
Figure 2: The normal material of the mixed nerve conduction velocity (CV)
of the median nerve at the forearm (from wrist to the cubital area, used at
Jorvi Hospital, N=72 normal subjects). A. CV values in different ages. B. The
response amplitudes (with Ln correction) in different ages. The values of our
patient are marked to the Figures (the orange square). The measurements
demonstrate the slowed CV but normal amplitude of the response.
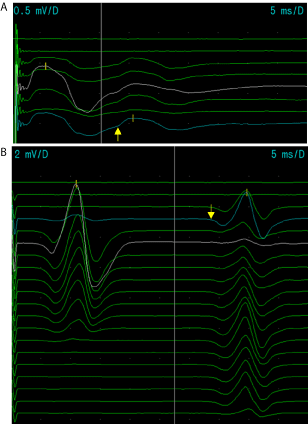
Figure 3: A. The H-reflex of the right median nerve of the patient in the first
ENMG study. The latency (arrow) is slowed, 18.2 ms, compared to the lengthcorrected
normal value (thin cursor).
B. The H-reflex of the right tibial nerve of the patient in the first ENMG study.
The latency (arrow) is slowed, 32.8 ms, compared to the length-corrected
normal value (thin cursor).
The reflex responses are of normal amplitude and elicited normally with
submaximal nerve stimulation. The studies of efferent motor axons (motor
CVs, F-responses and needle EMG) were all normal. This fact points out that
the slowing of the H-reflex corresponds to the slowing in 1a afferent nerve
fibers.
The last neurological study was performed 8 years after the onset of symptoms in August, 2016. The patient told that the symptoms were still going on: Disorders of balance, fatiguability of the hands and neck, as well as muscle twitches and cramps. However, there was no progression of symptoms. In the clinical neurological study Rombers´s sign was still pathological and tandem walking backwards was impossible. Muscle strength was in general slightly, but symmetrically decreased (4/5). In October 2016, ENMG study showed a similar 1a afferent slowing as the previous studies (Table 2). Somatosensory evoked potential study of the median nerve showed increased latency of the responses at Erb´s point (11.5 ms; p=1.52 %) and at the contralateral somatosensory cortex (N20 response 21.6 ms, p=0.95 %; p value is the percentage of normal people which could reach the value measured according to the height-corrected normal values of the laboratory; the limit of normality is 2.0 %).
1
norm
2
3
4
Medianus mot
52.7
≥49a
51.6
54.6
52.7f
Medianus F
28.0
≤28b
28.2
28.0
27.7f
Peron prof mot
45.2
≥39a
43.9
45.4
45.0
Peron F
50.7
≤52b
50.9
49.9
51.4
Med mixed nerve dx
56.1
≥63c
55.6
57.9
57.1
Med sens 3. finger
54.3
≥48d
51.9
54.7
54.5
Radialis sens dx
54.1
≥51d
57.8
62.5
60.9
Suralis sens dx
52.2
≥39d
52.2
53.0
53.7
Peron superf sens
48.1
≥36d
49.0
46.6f
46.6
H-refl n. tibialis
33.2
Z=44e
33.3
32.8
34.6
H-refl n. medianus
18.0
Z=32e
17.9
17.9
18.0
a) Mot=motor conduction velocity (m/s), mean of the right and left [12].
b) F=minimum F latency (ms), mean of the right and left [13].
c) Med mixed nerve: the conduction velocity of the 1a-afferent Aα nerve fibers in the right forearm (m/s) [6]. The normal values of Jorvi Hospital are expressed in Figure 2.
d) Sens: sensory conduction velocity (m/s), mean of the right and left for the median and superficial peroneal nerves, the other nerves in the right side. Compared to the normal material used in the laboratory.
e) H-refl=minimum latency of the H-reflex in submaximal stimulation (ms), mean of the right and left. Z-score, normal limit≤2.5; Normal values of the laboratory.
f) Measurement is only from the right side.
Red (cursive): pathological values. Time points 1: 10.2.2011, 2: 17.10.2012, 3: 8.10.2012, 4: 11.10.2016.
Table 2: Follow up of the neurography.
Discussion
The patient was a dental technician, previously healthy, and working full-time. Medical history revealed that 3 months before symptom onset she had received an Imovax vaccination for poliomyelitis. The neurological symptoms began after a 3 day fever in July 2008. The disease became chronic and caused permanent disability and decreased the patient’s working hours. During the following years regular exercise allowed some relief.
From the beginning the main symptoms were dizziness and disorders of balance. Moreover, after physical exercise and during night-time she experienced muscle cramps that tended to spread to neighboring muscles: for example during water running the cramps started at first in the toe muscles and then spread into more proximal leg muscles, until the patient had to stop the exercise. In addition, there were at times peculiar dysesthesias occurring during daily activities and even at rest resembling feelings of a powerful muscle tension, sudden electric shocks ora feeling of spontaneous distortion of the trunk and limbs. Later on, the patient also experienced increased fatigue of the muscles.
The findings in the neurological examination were slight: The Romberg´s sign was pathological, impaired balance with swaying and falling to the right or back when the eyes were closed. But with eyes open the patient was able to stay, even for a longer period, on either leg.
A distinct finding was detected in all four ENMG studies performed, but only in variables reflecting the function of the 1a afferents (Table 1) (Figure 2 and 3) [6,7]. The median nerve somatosensory evoked potential (SEP) latencies were also increased at the Erb´s point and cortically. These responses reflect primarily the function of 1a afferent nerve fibers; because they have a faster conduction velocity than the A beta or motor nerve fibers do [6]. All other nerve conduction values and also needle EMG were normal. It is possible, that also the responses of beta nerve fibers may have played a role in SEP latencies because the alpha fibers were slowed down in this patient. As the first ENMG study was performed as late as 2.5 years after the symptom onset the nerve conduction values at the acute phase were lacking. The measured values were practically similar in all the four ENMG studies during the follow-up time.
We may ask how the findings in ENMG correlate with the neurological symptoms of the patient. In all four ENMG studies there was a permanent slowing of the 1a afferent nerve fibers (Table 2), but no sign of axonal damage was evident. When there is an axonal damage on the 1a afferent nerve fibers, the amplitude of the median mixed nerve response diminishes and the H-reflex responses disappear because of the afferent nerve fiber paucity. However, in the patient both H-reflexes and myotatic reflexes were brisk, even though the H-reflex latency was increased. The neurological symptoms in the lower extremity may be explained by a poor timing: there was an essential delay in the delivery of the proprioceptive information from muscles to the central nervous system compared to normal situation. The delayed proprioceptive messages of the foot muscles may explain the impairment of balance and altered Romberg´s sign, and slowing of the afferent nerve impulses of the neck and back muscles may explain the dizziness. The upper cervical muscles and their proprioceptive afferent activity are important in the appreciation of the position of the head in the gravity field, and in overall balance control.
On the other hand the patient also expressed symptoms which may be connected to the hypersensitivity and increased spontaneous activity of 1a sensory receptors in muscle spindles. The patient described peculiar dysesthesias such as feelings of powerful muscle tension and sensations that the limbs and trunk are spontaneously distorted to peculiar positions, which they were not. These dysesthesias may be explained by spontaneous activation of the proprioceptive sensory afferents. The tendency for the extensive muscle cramps spreading into different muscle compartments may be explained by the hypersensitivity of the proprioceptive afferents that can strengthen the persistent inward current (PIC) of the respective motor neurons of the spinal cord. PIC mechanism normally increases the strength of the active muscles and this happens in part by the increased peripheral proprioceptive activation [7]. The central activation of PIC takes place via the noradrenaline and serotonin pathways [7]. Consequently, hyperactivity of PIC may lead to strong cramps, and a typical phenomenon in these cramps is the tendency to spread to the neighboring muscles, as the patient described during the water running exercise.
The primary reason for the demyelination of the 1a afferent nerve fibers was not found, but the involvement of the central nervous system was excluded by MRI imaging and by extensive serum and cerebrospinal fluid examinations. There was evidence of a rapid recovery, but the persistence of neurological symptoms was in line with the repeated ENMG findings demonstrating slow conduction in 1a afferents. In our patient we could follow only the sequelae of the primary disease, which took place for more than 2.5 years before the first ENMG study. It can be assumed that the values of the 1a afferent nerve fibers had been more pathological at the earlier stages of the disease.
Dysimmune neuropathy may selectively affect a certain structure of the axon or nerve sheath [8], such as the myelin sheaths or nodes of Ranvier of the 1a afferent nerve fibers. There are very narrowly targeted autoimmune neuropathies, for example acute motor or sensory axonal neuropathy (AMAN, ASAN), as well as multifocal motor neuropathy and motor conduction block neuropathy [9,10]. However, previous literature does not report any dysimmune neuropathy targeting only to the myelin sheath of the 1a afferent Aα sensory fibers. In order to recognize this entity, we would like to suggest that mixed nerve conduction velocity of the median nerve as well as the H-reflex measurements should be a part of ENMG studies for the accurate diagnostics of specific polyneuropathies.
References
- Dyck PJ, Thomas PK. Peripheral Neuropathy Vol 2. 4th edn. Philadelphia: Elsevier Saunders; 2005.
- Kikkawa Y, Kuwabara S, Misawa S, Tamura N, Kitano Y, Ogawara K, et al. The acute effects of glycemic control on nerve conduction in human diabetes. ClinNeurophysiol. 2005; 116: 270-274.
- Töyry JP, Partanen JSV, Niskanen LK, Länsimies EA, Uusitupa MI. Divergent development of autonomic and peripheral somatic neurpathies in NIDDM. Diabetologia 1997; 40: 953-958.
- Capasso M, Notturno F, Manzoli C, Uncini A. Involvement of sensory fibres in axonal subtypes of Guillain-Barré syndrome. J Neurol Neurosurg Psychiat. 2011; 82: 664-670.
- Dumitru D, Amato AA, Zwarts MJ. Electrodiagnostic Medicine, 2nd ed. Philadelphia: Hanley &Belfus Inc; 2002.
- Metso AJ, Palmu K, Partanen JV. Compound nerve conduction velocity- a reflection of proprioceptive afferents? Clin Neurophysiol. 2008; 119: 29-32.
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle & Nerve. 2005; 31: 135-156.
- Franssen H, Straver DCG. Pathophysiology of immune-mediated demyelinating neuropathies – part 1: Neuroscience. Muscle & Nerve. 2013; 48: 851-864.
- Franssen H, Straver DCG. Pathophysiology of immune-mediated demyelinating neuropathies – part 2: Neurology. Muscle & Nerve. 2014; 49: 4-20.
- Latov N. Diagnosis and treatment of chronic acquired demyelinating polyneuropathies. Nat Rev Neurol 2014; 10: 435-446.
- Fournier E, Viala K, Gervais H, Sternberg D, Arzel-Hézode M, Laforêt P, et al. Cold extends electromyography distinction between ion channel mutations causing myotonia. Ann Neurol. 2006; 60: 356-365.
- Chen S, Andary, Buschbacker R, Del Toro D, Smith B, So Y, et al. Electrodiagnostic reference values for upper and lower limb nerve conduction studies in adult populations. Muscle&Nerve. 2016; 54: 371-377.
- Benatar M, Wuu J, Peng L. Reference data for commonly used sensory and motor nerve conduction studies. Muscle&Nerve. 2009; 40: 772-794.
- Bear FM, Connors BW, Paradiso MA. Neuroscience, exploring the brain. 2nd ed. Baltimore: Lippincott Williams ja Wilkins; 2001.