
Special Article - Neurorehabilitation
Austin J Clin Neurol 2017; 4(3): 1111.
Plasma Growth Factors in Neuronal Regeneration
Rubio Jesús A*
Coordinator of the Unit of Hematology in Union Hospital of Murcia, Spain and the Regenerative Therapy Unit of the Miraculous, Spain
*Corresponding author: Alcaraz Rubio Jesús, Coordinator of the Unit of Hematology in Union Hospital of Murcia, Murcia, Spain and the Regenerative Therapy Unit of the Miraculous, Madrid, Spain
Received: May 07, 2017; Accepted: May 30, 2017; Published: June 15, 2017
Abstract
In mammals, the injured axons of the nerves do not regenerate. Often the functional recovery is incomplete. There is growing evidence in both preclinical and clinical studies that indicate that autologous growth factors have an important potential adjuvant therapeutic. Through the feedback of complex biochemical regulators that involve numerous cytokines, injured cells have specific receptors for these proteins involved in apoptosis and anti apoptosis that regulate both their own life cycle as the capacity of cellular differentiation. Recent studies have also observed the possibility of improving the levels of certain growth factors of the plasma depending on the enrichment in the final concentrate with platelets or mononuclear fraction. However, there are other fields of application in medicine, with new expectations, how is the neuro endocrine and neurorehabilitation, where infused of local or systemic way have the capacity to immunomodulation and chemotaxis of neuronal cells. It has also been shown in patients with degenerative neurological diseases (for example, Alzheimer’s Disease, vascular encephalopathy, multiple sclerosis, amyotrophic lateral sclerosis, hypoxic encephalopathy and anoxia), the plasma levels of various growth factors are lower than the values of reference, so that there is the hypothesis that might interfere with the mechanism of cellular hypoxia, producing both a function of neuroprotection, regeneration and differentiation of neural tissue.
Keywords: Plasma growth factors; Neurogenesis; Apoptosis; Angiogémesis; Anti-inflammatory; Neuro generation; Nerve repair
Introduction
Each year, about 2,570,000 patients are affected by various kinds of neuronal injury, which represents US$ 1,200 million per year on health care. Different types of mechanisms: metabolic, ischemic stroke, mechanical, thermal, can cause structural lesions in the nerve, gap, or neuropathy associated with a profound impact on the autonomic, sensory and motor function in patient [1,2]. Currently both microsurgical repair and transplantation of nerve autograft, together with the specific programs of neurorehabilitation is the gold standard treatment designed to increase the intrinsic potential regenerative of damaged axons. However, these treatments fail to recreate the appropriate cellular and molecular microenvironment of nerve repair, as well as the case of the auto grafts can produce a second iatrogenic injury in the affected area, increasing the patient morbidity. In spite of the innate ability of the adult mammalian to regenerate neurons, the functional recovery of nerve injury is often incomplete, leading to pain and disability in daily life and work activities. Several factors complicate the nerve regeneration that occurs naturally; among them: the type and mechanism of the lesion, the age of the patient, the proximity of the lesion to the cell body, and the atrophy in both distal Schwann cells and de nerved muscle tissue [2,3].
In recent years, the manufacture of scaffolding to guide the nerve regeneration, through therapies based on cellular biochemical and electrical signals, produced by growth factors, have attracted the attention of the researcher with the aim of improving the functional results to repair the nerve. Evidence is accumulating in both preclinical and clinical studies that indicate that the plasma rich in growth factors (PRP) constitute an important potential therapeutic not only as a neuroprotective effect, but intervening in biochemical processes of Neurogenesis and therapeutic modulation of inflammation, promoting the recovery of sensory and motor nerve and the muscle [4-6] (Figure 1).
Figure 1: Effects of Growth factors in neural tissue.
What are the Plasma Growth Factors?
Physiology of platelets and growth factors
Platelets are enucleated cell fragments derived from the cytoplasm of megakaryocytes in the bone marrow. Traditionally the best known function is in the process of primary hemostasis, as they are essential for clot formation, but also play an important role in inflammation, immunity, tumor progression and course thrombosis. By electron microscopy, it shows that platelets contain various organelles: mitochondria, ribosomes peroxisomes, and glycogen granules, the latter are divided into three types: 1) Alpha : Von-Willebrand containing fibrinogen, factor, platelet derived growth factor, ectodermal growth factor, vasculo-endothelial growth factor, insulinlike growth factor 1 and other growth factors, as can be seen in Table 1, 2 dense delta : that containing ADP, ATP, serotonin, epinephrine, norepinephrine and dopamine and 3) lambda : they are lysosomes, which help dissolve the clot once it has fulfilled its function. Similar Vesicules have been found in lysosomes at cytoplasm of leukomononuclear cells.
Content
Feature
Chemokines, cytokines
Platelet Factor 4
B-tromboglobina
RANTES*
macrophage inflammatory protein 1-alpha
Interleukin 1 and 8
Regulation of inflammation, chemotaxis
adhesive proteins
1 and 2 Thrombosin
Fibrinogen
Fibronectin
cell interactions and coagulation
Growth
Platelet derived growth factor (PDGF)
Transforming growth factor b (TGF-B)
epidermal or epithelial growth factor (EDGF)
Vaculo endothelial growth factor (VEGF)
Insulin-like growth factor-1 (IGF-1),
hepatocyte growth factor (HGF),
Brain-derived neurotrophic factor (BDNF)
cell proliferation and differentiation, chemotaxis, angigenesis, extracellular matrix synthesis
Immunoglobulins G
Ig-A, Ig-E, Ig-M and Ig-G
Immunological
Clotting factors V and VIII
thrombin production
Von-Wilebrand factor
platelet adhesion to subendothelial collagen
plasminogen activator inhibitor
Inhibition of fibrinolysis
P-selectin
leukocyte-platelet interaction
Table 1: Summary of the proteins in the platelet alpha granules.
Besides the classic functions described in platelets, recent discoveries in their ability to protein synthesis, mRNA containing copies of almost 1/3 of known proteins in the human genome, despite the lack of core, you have totally changed the perception they had of them, recognizing their ability to synthesize proteins to changes in their environment. Also being investigated are also some nongenomic functions of these factors, such as its effect on signaling pathways involving platelet activation and its role in de novo synthesis of both pro-and anti-inflammatory factors. The enormous amount of growth factors contained in platelet alpha granules, the ability to de novo synthesis of proteins and their microbicidal and modulating inflammation activity, favor the proliferation and cellular immunomodulator and synthesis of extracellular matrix, promoting healing, geriatrics and other tissue damage. These functions are precisely those that have led to propose the use of autologous plateletrich plasma for the repair and regeneration of various tissues [5,7].
The major growth factors of the most known function are:
PDGF (platelet-derived growth factor origin): Its main function is to indirectly promote angiogenesis via macrophages by a mechanism of chemotaxis. Activates macrophages, has a significant mitogenic activity on mesenchymal cells as well as neurons, microglia cells, promoting the proliferation of oligodendrocytes and remyelination and facilitates the formation of type 1 collagen.
TGF-beta (transforming growth factor-beta): Its main mission is to chemotaxis. Induces proliferation and differentiation of mesenchymal cells. It promotes collagen synthesis by osteoclasts. It is pro-angiogenic tissue, inhibits osteoclast formation and Proliferation of epithelial cells in the presence of other factors. Induces differentiation of neural stem cells.
FGF (fibroblast growth factor): Enables the proliferation and differentiation of osteoclasts, fibroblasts and induction of fibronectin by these neural stem cells and stem. Inhibit osteoclast action. It is an important pro-angiogenic action chemotactic activity on endothelial cells.
IGF-1 (insulin-like growth factor 1): It induces the proliferation and differentiation of mesenchymal cells and like coating has a potent mitotic effect on neural progenitor stem cellularity. It facilitates the synthesis of osteocalcin, alkaline phosphatase and type 1 collagen by osteoblasts.
VEGF (Vasculo endothelial growth factor): Enables chemotaxis and differentiation of endothelial cells, it promotes blood vessel air permeability.
Ectodermal growth factor (EGF): Great proapoptotical capacity, chemotaxis and differentiation of epithelial cells, renal, neural, glial and fibroblasts.
BDNF (brain-derived neurotrophic factor): induces the proliferation, differentiation and neuronal chemotaxis, microglial and oligodendrocitarial cellularity and remyelination thereof.
HGF (hepatocyte growth factor): Its main function of cell proliferation and differentiation, chemotaxis, angiogenesis and extracellular matrix synthesis
In Table 1 shows concisely the types of growth factors obtainable in platelet rich plasma and its main physiological function of the tissues. Likewise in Table 2 reflect normal levels of growth factors that can be found in human blood plasma of peripheral blood compared to average achieved in to platelet rich plasma quality.
Peripheral Blood
PRP
PDGF-AB (10-50 pg/ml)
45 pg/ml
360 pg/ml
TGF-B1 (10-70 pg/ml)
35 pg/ml
320 pg/ml
VEGF (15-85 pg/ml)
55 pg/ml
560 pg/ml
IGF-1 (0,5-19.5 pg/ml)
13 pg/ml
175 pg/ml
PLATELETS
(150.000-350.000/mm3)
265,000/mm3
1,250.000/mm3
LEUCOCYTES
(3.200-9000/mm3)
5,600/mm3
20,000/mm3
GRANULOCYTES
60% (3,330/mm3)
24% (480/mm3)
MONONUCLEARS
35% (1,960/mm3)
70% (14,000/mm3)
CD 34+
0.5/mm3
175/mm3
Table 2: Levels of growth factors and cell count in peripheral blood and PRP.
Definition of platelet rich plasma
The PRP is an autologous concentration of platelets in a small volume of plasma which represents an increase over normal baseline platelet levels, making it a source of easy access to growth factors contained therein. It has a pH between 6.5 and 6.7. It comes from the patient’s own blood, so it is free of communicable disease and can’t cause hypersensitivity reactions. The platelet count of the PRP is optimal debatable. According to the Competent Authority, it must contain to levels of platelets higher than the basal serum levels considered normal (between 200.000 and 450.000 platelets/mm3). But increasingly the authors dedicated to this area considered to PRP quality when platelet counts obtained in the final product exceeds 1,000,000 mm3. Alcaraz, et al. have demonstrated to prevalence of VEGF and TGF-b growth factors in those enriched leukocyte PRPs, while platelet- PRPs, leucocytes without have higher concentrations of growth factors type PDGF-AB and IGF-1 [4-6].
The Scientific Basis for the Usefulness of the Growth Factors Plasma (PRP) in Nerve Regeneration
The PRP administered locally, intraneural/perineural or systemic way, causes a release of cellular signaling molecules (Neural growth factor-NGF, Brain derived growth factor-BDGF), as well as growth factors, including NGF, Platelet derived growth factor -PDGF, Vasculo -endothelial growth factor- VEGF, Insulin like 1 growth factor - IGF-1 or Transforming growth factor B- TGFB alone or in combination that have been shown to exert a neuroprotective effect and anti apoptotic both on the neuron and on the adult neural stem cells, intervening in the repair of neural tissue. In this sense, growth factors, constitute the perfect support which facilitates the survival and neuronal differentiation, in such a way that infused on the brain tissue increase the viability and biological activity of the neural stem cells [2,3,7].
In addition, has been described in a murine model of bilateral cavernous nerve injury that infitration of Autologous growth factors had a neuroprotective and antifibrotic effect, facilitating nerve regeneration. In a recent in vitro study, neurons in mouse model of Alzheimer’s disease was showed that neurotoxicity induced by the aggregates of amyloid-b was significantly reduced after the infusion of growth factors probably mediated by the activation of the PI3K/ Akt anti apoptotic signaling pathway [2].
Despite the fundamental role played by the blood vessels in the axonal development through the site of the injury, there is strong scientific evidence that PRP promotes angiogenesis in bones, tendons, and muscles. We do not yet have enough studies to evaluate effect of angiogenesis in the nerve repair. Borselli, et al. showed in a ischemic limb mouse model with a loss of the neuromuscular junction that the administration of autologous VEGF and IGF-1 accelerates the regeneration of the neuromuscular junction with the increase of angiogenesis. In this sense, intramuscular injections of PRP would increase the angiogenesis and improve reperfusion after the induction of a severe skeletal muscle ischemia [2,3,7].
The crucial role played by the growth factors (PRP) has been showed in a rat brain model, where application of PRP promoted both the increase in the number and the growth of axons. This effect was significantly suppressed when added antibodies against these growth factors. The PRP has been used in a model of acute cerebral nerve injury in rabbits, as a culture medium to mesenchymal stem cells from bone marrow or neurological stem cells reporting beneficial effects on axonal count, Myelination and electrophysiological functionality. An example about the use of PRP as a filling of nerve grafts, shown in the work of Zheng, et al. who demonstrated a dose-dependent effect of PRP in sciatic nerve injury using auto grafts and PRP in a rat model facilitating the proliferation, migration and the neurotrophic function of rat mesenchymal stem cells cultured with PRP, reporting significant improvements in the diameter, thickness, myelination and number of axons, as well as a recovery of the electrophysiological parameters. These results are consistent with the work of Kaplan, et al. who used PRP gels as a filling of collagen, communicating the improvement in the structural and functional results in a model of sciatic nerve injury in rat. Seddon applied PRP in a suturable membrane to wrap the neurorrafia in a model of acute sciatic nerve injury, showing a strong signal of EMG, due to a significant higher of axonal density at the site of the injury. In this sense, two studies reported the positive effects of the use of PRP as adjuvant in the suture of the nerve. Farrag, et al. reported that PRP could increase the thickness of the myelin and amount of axons when the injured nerve is stitched with local application of PRP; while Sariguney, et al. showed an increase in functional activity at the date of latency associated with improvement in the thickness of the myelin. Sánchez, et al. conducted a study in sheep using PRP as a filling of the damaged nerves to coat the plasty of the nerve, and reported an increase in the electrophysiological response showed a greater axonal density, reducing the muscle atrophy in the treated animals compared with the saline solution or the spontaneous regeneration of the other groups. Therefore, the PPR could contribute significantly to the two key events for a proper axonal regeneration: angiogenesis and the establishment of an optimal microenvironment for the differentiation, immunomodulation and cell division [2,3,5,6]. This fact can be seen in Figure 2.
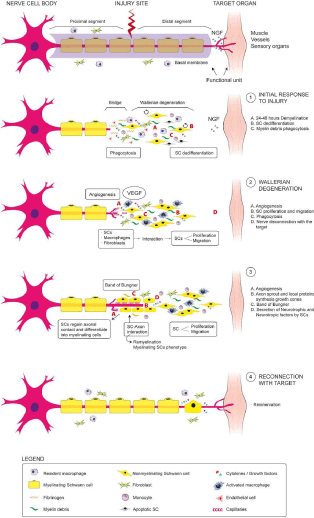
Figure 2: Levels of growth factors and cell count in peripheral blood and PRP.
On the other hand Anitua, et al. reported an anti-inflammatory powerful activity of PRP, aiming that b-amyloid expresses cytokines inhibited when astrocytes are cultivated with autologous growth factors. This phenomenon could be explained by suppression of NFkB in astrocytes. In a mouse model of Parkinson’s disease, Anitua et al. showed that the neuro inflammatory process or gliosis, mediated by microglia, was reduced by PRP, improving motor performance, associated with a strong reduction of nuclear transcription factor kB (NF-kB) and nitric oxide, along with the activation of the Cyclooxygenase and the expression of tumor necrosis factor in the brain; in line with results of other studies which report that plasma growth factors like IGF-1, PDGF and TGFB, could inhibit the NFkB on the tenocytes, synovial cells, fibroblasts, chondrocytes and change the macrophages from phenotype M1 to M2. In a rabbit model in which was induced injury to the median nerve with dextrose was demonstrated that the infiltration of PRP in the carpal tunnel produced a significant reduction of the swelling in nerve, compared with control group at 4 weeks of follow-up of the injury [2,6,7].
The growth factors would produce a phenomenon of neurogenesis through 3 ways: First inhibiting the inflammatory phenomenon or irreversible gliosis that would difuult the anatomical and functional neuronal recovery. Secondly facilitating the migration and proliferation of stem trunk neuronal cells at the site of the lesion and finally stimulating its differentiation towards mature neuronal mass reestablishing the normal functional circuit of the same.
The lesion of a neuron mobilizes Macrophagues and Monocites that phagocyte the myelin residues, stimulating in a autocrine way the nerve growth factor (NGF), which facilitates the recruitment Schwann cells to the area of the lesion, facilitating their differentiation and proliferation together with the vasculo endotelial growth factor producing remyelination and final reconnection of the affected axon.
The use of plastias as scaffolds enriched with autologous growth factors, as a bridge between the ends of an injured nerve, allows the re innervation and the gradual functional recovery of the same due to the implementation of new axonal mass
Improvement in the Neurological Parameters in Humans
PRP has been applied as a fill in post-traumatic injuries of nerves, such as dynamic scaffold concentrate infiltrated peril or intraneurally, or both, as in the case of a peroneal nerve palsy and other damaged nerves with beneficial results in terms of better functional recovery. Kuffler reported functional recovery in patients under 58 years old whose nerve deficits of 2-16 cm were treated with collagen tube filled with PRP at 0.5-3 years after the trauma [2,6-8] (Figure 3).
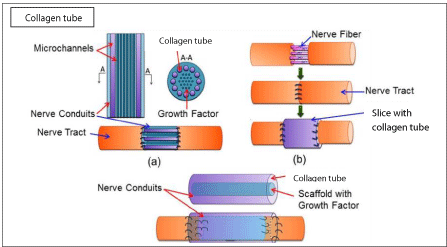
Figure 3: Effect of growth factors on platias grafts in the injured nerve.
In a double-blind randomized clinical trial, the implementation of the perineural injections of PRP in the tibial and ulnar nerve, showed improvement of the sensory neuropathy in patients suffering from leprosy. In a retrospective analysis in 10 patients with persistent pudendal neuralgia was injected PRP activated with calcium gluconate around the nerve affection, reporting a significant reduction of pain. In a case series of 14 patients with carpal tunnel syndrome, a single injection guided by ultrasound around the median nerve led to the disappearance of the pain in 8 patients, and relieving pain in 3 patients at 3 months follow-up. Sanchez, et al. in a series of clinical cases that the application of sequential perineural and intraneural injections of PRP at proximal and distal level in a paralysis of the peroneal nerve reported a significant functional recovery, seeking electromyographic signs of re innervation in peroneal, longus and tibialis anterior muscles that traduced in a full functional recovery. It has been reported by Alcaraz and col that the intravenous injection of 25 cc of PRP concentrates in a child of 6 years old with severe perinatal Cerebral Palsy is safe, with an increase of cortical level carbohydrate uptake using PET brain at 6 months follow up, resulting in a considerable improvement both cognitive sphere and language. This fact was confirmed by Sánchez López J, et al. through a case-control study in 25 patients suffering of severe cerebral palsy, in who coadyuvation both an intravenous infusion of 25cc of PRP rich in Leukocyte growth factors and specific program of Neurorehabilitation produced a remarkable improvement in the cognitive sphere, objectified by Barthel index, reducing recovery times in affected patients at 2 months after treatment, statistically significant (p<0.003), compared with the control group [2,5-9].
Conclusion
The application of PRP both locally or systemically is a feasible option, safe, with a high power of neuroprotection and neuro regeneration, which traduces into an extraordinary improvement in the sensorial-analgesic, motor and cognitive functionality in the patient. However, still are necessary studies with a greater scientific power in the form of clinical trials to analyze this effect, given that the majority of existing studies in humans are based on series of clinical cases without the existence of control groups.
References
- Harguindey S. Apoptosis y antiapoptosis en cáncer, Alzheimer y procesos neurodegenerativos: una dialéctica de contrarios? Nuevo abanico de posibilidades terapéuticas y peligros potenciales. Oncologia. 2004; 27: 579- 589.
- Sánchez JM, Alcaraz J, Oliver A. Plasma rich in Leukocyte growth factors in patients with cerebral palsy: a case control study. International Journal of Recent Advances in Multidisciplinary Research Vol. 03, Issue 06, pp.1513- 1521, June, 2016.
- Brea D, Sobrino T, Ramos P, Castillo J. Reorganización de la vascularización cerebral tras la isquemia. Rev Neurol. 2009; 49: 645-654.
- Alcaraz J, Oliver A, Sánchez JM. Nuevo método de obtención de plasma rico en factores de crecimiento plaquetario (PRP), Estudio descriptivo en 15 pacientes y comparación con los resultados publicados en la bibliografía. Rev Hematol Mex. 2015; 16: 210-216.
- Oliver AJ, Sánchez A, Lajara JJ. Clinical use of Platelet-Rivh Plasma: A new dimension in Regenerative Medicine. Med Sci Rev. 2015; 111-120.
- Legido A, Valencia I, Katsetos C, Papadopoulos M. Neuroprotección en la encefalopatía hipóxico isquémica perinatal. Tratamientos con eficacia clínica demostrada y perspectivas futuras. Medicina (Buenos Aires). 2007; 67: 6-1.
- Alcaraz J, Oliver A, Sánchez JM. Platelet-Rich Plasma in a Patient with Cerebral Palsy. Am J Case Rep. 2015; 16: 469-472.
- Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Aurologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004; 91: 4-15.
- Sánchez M, Anitua E, Delgado D, Sanchez P, Prado R, et al. Platelet-rich plasma, a source of autologous growth factors and biomimetic scaffold for peripheral nerve regeneration. Expert Opin Biol Ther. 2017; 17: 197-212.