
Case Report
Austin J Clin Neurol 2017; 4(5): 1121.
A Case Report of Neuroglycopenic Coma with Diffuse Cortical Involvement
Phani Kumar P*, Divya Latha Y, Ramadevi M and Jaya Bhaskar C
Department of General Medicine, Sri Venkateswara Medical College, India
*Corresponding author: Phani Kumar P, Department of General Medicine, Sri Venkateswara Medical College, Tirupati, Andhra Pradesh, India
Received: June 09, 2017; Accepted: July 10, 2017; Published: July 26, 2017
Abstract
Hypoglycemia is a metabolic abnormality that triggers a series of physiological, psychological and behavioral responses. Prolonged hypoglycemia can result in neuronal dysfunction and death. A 31year old male patient presented with hypoglycemia and altered sensorium. MRI brain showed bilateral hyper intense areas with gyral edema in fronto parietal cortex with sparing of deep grey nuclei, viz, cerebellum, brainstem, basal ganglia, capsular tracts and thalami, suggestive of neuroglycopenic coma. With early identification and prompt treatment, hypoglycemia can be reversed, otherwise is associated with poor prognosis.
Keywords: Neuroglycopenia; Hypoglycemia
Abbreviations
CVS: Cardiovascular System; RS: Respiratory System; GIT: Gastrointestinal System; CSF: Cerebrospinal Fluid; GCS: Glasgow Coma Scale; FLAIR: Fluid Attenuated Inversion Recovery; DTR: Deep Tendon Reflexes
Introduction
Hypoglycemia is a metabolic abnormality that triggers a series of physiological, psychological and behavioral responses. The clinical manifestations include neuroglycopenic symptoms such as inability to concentrate, drowsiness, confusion, speech abnormality and in coordination due to brain fuel deprivation and neurogenic or autonomic symptoms such as palpitations, tachycardia, diffuse weakness, anxiety and hunger [1,2,5]. Prolonged hypoglycemia can result in neuronal dysfunction and death, with deficits ranging from measurable cognitive impairments to aberrant behavior, seizures and coma [1,2]. In sustained hypoglycemia, the integrity of cerebral neurons is not preserved [3]. The initial changes are seen in mitochondria, first in dendrites followed by stoma. Ultimately there is rupture of cell membranes and cell death [4]. DWI of patients with acute hypoglycemia has shown high-intensity signals in numerous different locations of the brain. Although cortex, basal ganglia, and hippocampus seem to be the brain tissues most vulnerable to hypoglycemia, the underlying mechanism of the distribution pattern of the high-intensity signals remains unclear. Asymmetry of the lesions might be explained by the asynchronous onset of electro-cerebral silence between the hemispheres [4]. Involvement of neostriatum and diffuse cortical lesions are associated with poor outcome [5].
Case Presentation
A 31year old male patient brought to causality with loss of consciousness and seizures. Patient’s attenders gave history of consumption of alcohol and retired to bed the day before his presentation. Attenders noticed excessive snoring in the morning after few hours he developed seizures. He is brought in unconscious state to causality on the same day.
At the time of presentation GCS is 3/15. Vitals: pulse rate: 96 bpm, blood pressure: 90/60 mm of Hg, Spo 2:88% with room air and 96% with 4L of oxygen. On examination, CVS and GIT: normal, RS: Coarse crepitations present, CNS: Bilateral pin point pupils, not reacting to light, hypotonia with absent DTR in alllimbs. Planter’s bilateral no response. Investigations: Random blood sugar: 39 mg/dl, Total counts: 7,500 cells/mm³, differential counts: polymorphs 78%, lymphocytes 18%, Serum Creatinine: 2.3 mg/dl, serum electrolytes: Na+ 149 mmol/l, K+3 mmol/L, Cl-114 mmol/L. CT brain plain is normal. Patient diagnosed as Neuroglycopenia with aspiration pneumonia and treated accordingly.
On Day 2, GCS is 3/15, patient developed high grade fever, vitals: pulse rate: 102 bpm, blood pressure: 110/70 mm of Hg, temperature: 102 °F, SpO2: 94% with 4L of oxygen, CVS and GIT: normal, RS: Coarse crepitations present, CNS: Bilateral pin point pupils, not reacting to light, hypotonia with absent DTR in all limbs, neck stiffness and extensor plantar response observed. Fundus is normal. CSF analysis showed: Total counts: 5 cells/mm³, differential count: lymphocytes 100%, proteins: 20 mg/dl, sugars: 187 mg/dl, adenosine deaminase levels: 4.6 U/L.
On day 3, Patient Glasgow come scale is 3/15, vitals: pulse rate: 96 bpm, blood pressure: 90/60 mm of Hg, SpO2: 88% with room air and 96% with 4L of oxygen, CVS and GIT: normal, RS: Coarse crepitations present, CNS: Anisokoria is noticed. Pupil’s right- 5 mm, left- 1 mm not reacting to light. MRI brain done T2W, FLAIR images showed bilateral symmetrical hyperintense areas with gyral edema in frontoparietal cortex with sparing of deep grey nuclei, viz, cerebellum, brainstem, basal ganglia, capsular tracts and thalami, no evidence of hemorrhage or space occupying lesion in parenchyma suggestive of neuroglycopenia (Figure 1,2A,2B). The same day at 6 pm on examination anisokoria on being not reversed, after exclusion of space occupying lesion, pilocarpine drops (0.025%) were installed and after 45 minutes pupils were examined and constriction of right pupil was noted with no alteration in size of left pupil, indicating involvement of ciliary ganglion, probably due to chronic alcoholism. Patient couldn’t be revived and expired on day 4.
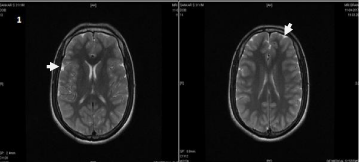
Figure 1: T2 FLAIR images showing diffuse gyral hyper intensity involving
entire cortex.
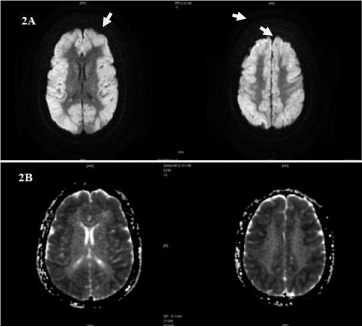
Figure 2A and 2B: DWI & Corresponding ADC images showing gyral
diffusion restriction involving entire cortex.
Discussion
Delayed treatment of hypoglycemia results in neurological sequelae like seizures, speech abnormalities, ataxia, stroke like symptoms and loss of consciousness [2,5,6-9].
Hypoglycemia was initially reported to predominantly involve the cortex, neostriatum, and hippocampus [10-12] many have reported predominant white matter involvement, mainly affecting the centrum semi ovale, corona radiata, internal capsule and splenium of the corpus callosum. In fact, involvement of the white matter is now thought to be earlier and more common than grey matter involvement. Some cases may show diffuse grey matter and white matter involvement [13]. The thalamus, brainstem and cerebellum are invariably spared and this may help to differentiate hypoglycemia from hypoxemic injury which often involves the thalamus [14].
On the basis of topographic distribution of signal abnormalities, three imaging patterns have been described [15]. These include 1) predominant grey matter involvement affecting the cortex, neostraitum, hippocampus. 2) predominant white matter involvement affecting the periventricular white matter, internal capsule and spenium of corpus callosum. 3) mixed pattern involving both grey and white matter.
In our case, MRI T2W FLAIR (Figure 1,2A,2B) shows subtle bilateral symmetrical hyper intense areas with gyral edema in fronto parietal cortex with sparing of deep grey nuclei viz, cerebellum, brainstem, basal ganglia, capsular tracts and thalami with mild diffuse cerebral edema.
The cerebellum is less prone to hypoglycemic insult; it may relate to greater efficiency of glucose transporter, thus making hypoglycemia induced cerebellar dysfunction a less common complication of severe hypoglycemia. Taguchi, et al. [16] reported the brain magnetic resonance imaging findings of a patient with hypoglycemic encephalopathy, which included increased reversible signal intensity in the left temporal and occipital gray matter, right putamen, both posterior limbs of internal capsule and triangular area of lateral ventricle [15]. Patients with focal involvement of internal capsule or corona radiata or splenium usually have good prognosis, [14] these lesions resolve promptly after restoration of blood glucose, though tend to follow clinical symptom resolution.
Patients with extensive white matter involvement show variable response. The prognosis in these cases varies between complete recovery and persistent vegetative state [4] Involvement neostriatum and diffuse cortical lesions portend dismal outcome [15]. Failure of lesions to regress on follow up imaging is also associated with poor prognosis.
Conclusion
Hypoglycemia is reversible if recognized and treated promptly, delayed recognition leads to neuroglycopenia with poor outcome. White matter involvement is most commonly observed in neuroglycopenia. Superficial grey matter involvement is also observed in neuroglycopenia. Involvement of superficial cortical structures will have dismal outcome.
References
- Frier BM. Hypoglycemia. In: Encyclopedia of Stress. 2007: 408-413.
- Cryer PE. Hypoglycemia, functional brain failure, and brain death. J Clin Invest. 2007; 117: 868-870.
- Adams D, Maurice V, Ropper AH. The acquired metabolic disorders of nervous system, Principles of Neurology. 6th edi. London: McGraw Hill; 1997. 1115-1116.
- Auer RN. Hypoglycemic brain damage. Metab Brain Dis. 2004; 19: 169-175.
- Graveling AJ, Frier BM. Hypoglycaemia: an overview. Prim Care Diabetes. 2009; 3: 131-139.
- McNay EC, Cotero VE. Mini-review: impact of recurrent hypoglycemia on cognitive and brain function. Physiol Behav. 2010; 100: 234-238.
- Lishman WA. In: Organic psychiatry the psychological consequences of cerebral disorder. 3rd ed. UK: Blackwell Science Ltd. 1998: 533-546.
- Frier BM. Morbidity of hypoglycemia in type 1 diabetes. Diabetes Res Clin Pract. 2004; 65: S47-S52.
- Chinnapongse RB, Odderson IR, Johnson RJ. Hypoglycemic coma associated with brain infarcts. J Stroke Cerebrovasc Dis. 1998; 7: 154-156.
- Berz JP, Orlander JD. Prolonged cerebellar ataxia: an unusual complication of hypoglycemia. J Gen Intern Med. 2008; 23: 103-105.
- Kang EG, Jeon SJ, Choi SS, Song CJ, Yu IK. Diffusion MR imaging of hypoglycemic encephalopathy. AJNR Am J Neuroradiol. 2010; 31: 559-564.
- Schmidt P, Böttcher J, Ragoschke-Schumm A, Mentzel HJ, Wolf G, Müller UA, et al. Diffusion-weighted imaging of hyperacute cerebral hypoglycemia. AJNR Am J Neuroradiol. 2011; 32: 1321-1327.
- Johkura K, Nakae Y, Kudo Y, Yoshida TN, Kuroiwa Y. Early diffusion MR imaging findings and short-term outcome in comatose patients with hypoglycemia. AJNR Am J Neuroradiol. 2012; 33: 904-909.
- Atay M, Aralasmak A, Sharifov R, Kilicarslan R, Asil T, Alkan A. Transient cytotoxic edema caused by hypoglycaemia: follow-up diffusion weighted imaging features. Emerg Radiol. 2012; 19: 473-475.
- Ma JH, Kim YJ, Yoo WJ, Ihn YK, Kim JY, Song HH, et al. MR imaging of hypoglycaemic encephalopathy: lesion distribution and prognosis prediction by diffusion-weighted imaging. Neuroradiology. 2009; 51: 641-649.
- Taguchi T, Mizobuchi M, Terada Y. Hypoglycemic encephalopathy as a result of an attempted suicide. Clin Neurol Neurosurg. 2010; 112: 455-456.