
Research Article
Austin J Clin Neurol 2019; 6(1): 1134.
Glutathione Redox Status in Neurodegenerative Diseases
Dellanoce C1, Fulgenzi A2 and Ferrero ME2*
¹CNR Institute of Clinical Physiology, Cardiothoracic and Vascular Department, Niguarda Ca’ Granda Hospital, Italy
²Department of Biomedical Sciences for Health, Università degli Studi di Milano, Italy
*Corresponding author: Maria Elena Ferrero, Department of Biomedical Sciences for Health, Università degli Studi di Milano, Italy
Received: March 28, 2019; Accepted: July 19, 2019; Published: July 26, 2019
Abstract
Oxidative stress is involved in the pathogenesis of many diseases, particularly Neurodegenerative Diseases (ND). Glutathione (GSH) is the most abundant endogenous antioxidant, as well as being a critical regulator of oxidative stress. This study was carried out to analyze levels of GSH and other redox active compounds, known as aminothiols, in patients affected by ND or by Fibromyalgia (FM). We also evaluated the influence of GSH treatment during chelation therapy.
We studied 40 healthy subjects and 41 patients affected by ND or FM. All underwent chelation therapy with calcium disodium ethylenediaminetetraacetic acid (CaNa2EDTA or EDTA) to remove toxic metal burden. We evaluated aminothiol levels (cystine, cysteine and glycine, homocysteine, and GSH) in the plasma and red blood cells of healthy patients and ND (Multiple sclerosis and Amyotrophic lateral sclerosis, Parkinson’s disease, Alzheimer’s disease) or FM patients.
We found GSH levels to be significantly lower in ND and FM patients than in their healthy counterparts, both in plasma and in red blood cells. Treatment of ND and FM patients with 250mg GSH daily for one month significantly improved GSH levels in red blood cells. Homocysteine levels were unaffected by GSH treatment, suggesting impairment of the transsulfuration pathway of homocysteine metabolism in ND patients.
Lower GSH levels are present in ND and FM patients compared to healthy controls, with a reduction of the endogenous antioxidant defense system. Oral GSH supplements during chelation therapy counteracted the depletion of endogenous GSH in these patients.
Keywords: Glutathione; Aminothiols; Neurodegenerative diseases; fibromyalgia
Abbreviations
ND: Neurodegenerative Diseases; GSH: Glutathione; FM: Fibromyalgia; EDTA: Ethylenediaminetetraacetic Acid; ROS: Reactive Oxygen Species; RNS: Reactive Nitrogen Species; MS: Multiple Sclerosis; ALS: Amyotrophic Lateral Sclerosis; PD: Parkinson’s Disease; AD: Alzheimer’s Disease
Introduction
The delicate balance in cellular redox is created on the one hand by the production of oxidants, such as Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS), and on the other through activation of the antioxidant systems that eliminate them. Both ROS and RNS, when produced at physiological values, can activate the specific signaling pathways required for many cellular functions, including cell growth and immune responses [1]. However, increased ROS/RNS production or decreased antioxidant capacity can cause oxidative/nitrosative stress [2]. In particular, at the level of synapsis and neuronal cells, imbalance between oxidant production and antioxidant mechanisms can contribute to neurodegeneration. Oxidative and nitrative stress play a critical role in the pathogenetic mechanisms of Neurodegenerative Diseases (ND), such as Multiple Sclerosis (MS), Amyotrophic Lateral Sclerosis (ALS), Parkinson’s Disease (PD), and Alzheimer’s Disease (AD). Glutathione (Gamma-L-Glutamyl-L-Cysteinyl-L-Glycine) (GSH) is a major component of the antioxidant system that defends cells against the toxic effects of ROS/RNS and provides a reducing environment within the cells. Neurons are the cells that are most vulnerable to ROS/RNS excess as they express antioxidants (scavengers and enzymes) at low levels; their survival relies on the antioxidant protection promoted by neighboring astrocytes [3]. Although GSH is widely distributed throughout human tissue, its concentration in neurons is lower than that found in astrocytes [4,5]. GSH is synthesized by the sequential action of the enzyme Glutamate-Cysteine Ligase (GCL) and GSH synthetase [6]. The heterodimer GCL, composed of two subunits, catalyzes the formation of gamma-glutamylcysteine, which represents the rate-limiting reaction in GSH synthesis. A chronic decrease in GSH content in GCLM knockout astrocytes can induce a response involving changes in protein expression and lysine acetylation [7]. There is much evidence to suggest that GSH depletion plays a role in the onset of ND. Indeed, transient GSH depletion in the substantia nigra compacta has been associated with neuroinflammation in rats [8]. Moreover, under conditions of chronic GSH decrease, moderate over-expression of wild-type Superoxide Dismutase (SOD1) in mice causes overt motor neuron degeneration, similar to that induced by ALS-linked mutant SOD1 over-expression [9]. In an in vitro model of nerve-cell death, GSH depletion by glutamate was potentiated using iron and copper, suggesting important implications for age-related ND [10].
Which arms can help regulate/improve GSH redox status? Some antioxidants have been proposed as GSH system modulators. Indeed, resveratrol, through the heme oxygenase pathway, is known to protect C6 astroglial cells against GSH depletion [11]. Curcumin treatment attenuates the inflammation, oxidative stress, and ultrastructural damage induced by spinal cord ischemia/reperfusion injury in rats, and in particular improves GSH peroxidase [12]. Many studies examine the modifications of GSH redox status in patients affected by ND. Improved oxidative DNA damage and DNA susceptibility to oxidation result from a diminished GSH/GSSG (oxidized glutathione) ratio in AD patients treated with or without memantine [13]. Furthermore, association between lower cortical GSH levels, brain amyloidosis, and reduced memory in healthy older adults examined with 1H-MR spectroscopy, suggests GSH measurement as a noninvasive biomarker for mild cognitive impairment and early AD [14,15]. During disease progression in ALS patients, a systemic pro-inflammatory state and an impaired endogenous antioxidant system (in particular GSH system) have been noted [16] and related to clinical status at diagnosis [17].
Treatment strategies in ND should aim to promote the reactivation and correct functioning of redox system balance. Treatment with N-acetylcysteine (to increase GSH) and selenium supplements (to protect against excessive exposure to copper and iron) has been proposed [18].
The rationale of the present study was to assess oxidative stress in the plasma and Red Blood Cells (RBC) of patients affected by ND or by Fibromyalgia (FM) undergoing chelation therapy by examining thiol levels (SH-) containing amino acids known as aminothiols, e.g. cystine, cysteine and glycine, homocysteine, and GSH. In particular, we verified whether ND and FM patients showed a lower GSH content compared to healthy controls and studied the effects of GSH treatment on the same patients.
Methods
Participants
Each patient elected to undergo chelation therapy to remove toxic metal burden. Out of 90 consecutive subjects who had undergone a medical checkup at an outpatient clinic, only the 81 that agreed to comply with the trial protocol were enrolled; they accepted to undergo chelation therapy once a week and take GSH daily. All patients received both chelation therapy and GSH treatment without randomization.
As reported in Table 1, we enrolled 41 patients (22 women and 19 men) affected by ND (3 ALS; 2 PD, 2 AD, 27 MS) or by FM (7). We also recruited as controls 40 (21 women and 19 men) patients not affected by any disease of note, but who had previously been exposed to environmental or occupational heavy metals. Age ranged from 18 to 84 years. Patients with early neurological diagnosis of ND or FM without mild cognitive impairment and controls spontaneously decided to undergo tests to verify their toxic metal burden and blood redox status. All subjects provided written informed consent. Declaration of Helsinki and all procedures involving human participants were approved by Milan University’s Ethical Advisory Committee (number 64/14). Further inclusion/exclusion criteria were also applied. Indeed, some patients had never previously been treated for ND or FM with conventional drugs due to the recent onset of the pathology. The ND patients involved were mostly affected by MS; ten of them had previously been treated with conventional drugs (e.g. immunosuppressant agents), but had spontaneously interrupted therapy for approximately 3 months before the beginning of the present study.
Male
Female
Age
TOTAL
(M/F)
Controls
19
21
48/47
40
ND
19
22
48/42
41
MS
11
16
47/40
27
AD
1
1
53/43
2
ALS
3
0
33
3
FM
1
6
60/46
7
Table 1: Characteristics of enrolled subjects.
Study design
The aim of this prospective study was to evaluate the influence of GSH treatment in patients undergoing chelation therapy.
All subjects (ND, FM, and controls) underwent chelation therapy for 3 months. The chelating agent EDTA is endowed with antioxidant properties, yet as it was administered weekly, the participants were treated daily with GSH. Toxic metal burden was evaluated using the “chelation test”.
The chelating agent calcium disodium ethylenediaminetetraacetic acid (CaNa2EDTA or EDTA) (2 g), diluted in 500mL physiological saline (Farmax srl, Brescia, Italy), was slowly (over 2 hours) administered intravenously, and subjects were invited to collect urine samples before and after the first intravenous EDTA treatment. Urine collection following chelation treatment lasted 12 hours. Urine samples were carefully put into sterile vials and sent to the Laboratory of Toxicology (Doctor’s Data Inc. St. Charles, IL, USA) for analysis.
The first “chelation test” to show heavy metal intoxication induced our subjects to undergo weekly chelation therapy.
The redox state in plasma and in red blood cells was determined in both ND/FM patients and in controls (basal values). Successively, daily GSH treatment started. After 1 month and 3 months of treatment, further evaluation of the redox state in plasma and in red blood cells was carried out.
Sample collection: Three mL of whole blood was drawn from patients through venipuncture and transferred to test tubes containing EDTA. Plasma was immediately separated from blood cells by centrifugation at 4000 g for 2 minutes a t 4ºC to prevent thiol oxidation. Plasma was recovered and aliquots of RBC were transferred to test tubes and frozen in liquid nitrogen. Each sample was stored at -80ºC until analysis.
Laboratory assessments: Cystine (CYS), Cysteine and Glycine (CYSGLY), Homocysteine (HCY), and Glutathione (GSH) were determined in plasma and in RBC using the chromatographic method previously described and validated [19]. Briefly, tris- (2-carboxyethyl)-phosphine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) and 4-fluoro-7-sulfamoylbenzofurazan (ABD-F) (Sigma-Aldrich) were respectively used as reducing and derivatizing agents. After protein precipitation with trichloroacetic acid (Merck, Darmstadt, Germany), the samples were centrifuged at 14,000g for 10 min at 4°C. Clear supernatant (100 μL) was incubated for 90 min at room temperature with ABD-F before chromatographic analysis.
pThiol separation was performed using isocratic high-performance liquid chromatography analysis on a 5 μm Discovery C18 analytical column (250×4.6 mm i.d, Supelco, Sigma-Aldrich) at room temperature, eluted with a solution of 0.1 M acetate buffer (Merck), pH 4.0–methanol (Merck), 81:19 (v/v) at a flow rate of 1 mL/min. Fluorescence intensities were measured with excitation at 390 nm and emission at 510 nm using a JASCO fluorescence spectrophotometer (Jasco Europe, Cremella, Lecco, Italy).The results are expressed as μmol/L.
Patient treatment: Each patient was tested for aminothiol basal values. All patients received 250 mg/day of GSH (Oximix 7+, Driatec, Cassina De’ Pecchi, Milan, Italy) (1 capsule/day) for three months, and were tested for aminothiol levels at 1 and 3 months after the beginning of therapy. At study entry, and at 1 and 3 months after enrollment, blood samples were collected to ascertain liver function (aspartate aminotransferase, alanine aminotransferase, and gammaglutamyltransferase), kidney function (creatinine), blood lipids (total cholesterol, high density lipoproteins, low density lipoproteins), hemochromocytometric data and glucose (data not shown). All patients adhered to therapy.
Statistical analysis: Results were expressed as mean±Standard Deviation (SD) of mean. They were analyzed using t test. Statistical tests were two-sided, and significance was assumed at P<0.05. We used IBM SPSS Statistics.
Results
Compliance and adverse effects
Based on diary entries, compliance was good, with just ‹0.1% of scheduled doses missed. No adverse effects were reported by the study participants. Table 1 shows the characteristics of the enrolled subjects.
All patients were affected by toxic metal burden (data not shown) and underwent weekly chelation therapy.
Total and reduced aminothiols in plasma and RBC
Figure 1 shows the levels of CYS, CYSGLY, HCY and GSH as total aminothiols in Plasma (PT) or Reduced Aminothiols in Plasma (PR), and as total aminothiols in RBC (ET) or reduced aminothiols in RBC (ER) examined in controls and in ND/FM patients (all reported in the Figure as ND).
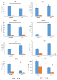
Figure 1: The figure shows total (PT) and reduced (PR) aminothiols in
plasma and total (ET) and reduced (ER) aminothiols in erythrocytes (RBC) in
healthy controls and patients affected by Neurodegenerative Diseases (ND).
The values are expressed as μM/L.
Cystine=CYS, cysteine and glycine=CYSGLY, homocysteine=HCY,
glutathione =GSH.
*=P‹0.05 compared to controls.
Figure 1 shows that while CYS PT levels were significantly lower in ND patients compared to controls, CYS ET levels were significantly higher. No significant differences were seen in CYS PR levels or in CYS ER levels between ND patients and controls. Analogously, CYSGLY PT levels were significantly lower in ND patients than in controls, whereas CYSGLY ET levels were significantly higher. No significant differences were seen in CYS PR or in CYS ER levels between controls and ND patients.
HCY PT and HCY ET levels were significantly higher in ND patients than in controls. HCY ER levels were undetectable in ND patients.
GSH PT and GSH PR levels were significantly lower in ND patients than in controls, as were GSH ET and GSH ER levels.
We have not reported separately the values related to the biggest group of MS patients compared with those of the other less numerous ND patients. Indeed, each patient affected by ND showed very low levels of GSH. Moreover, aminothiol contents in MS patients did not significantly differ from those in other ND patients.
Treatment with GSH did not significantly modify the levels of aminothiols in plasma and RBC in controls (data not shown).
Figure 2 shows the effects of 1-month daily GSH treatment on total (PT) and reduced (PR) aminothiols in plasma and total (ET) or reduced (ER) aminothiols in RBC of ND/FM patients (all reported in the Figure as ND).
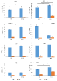
Figure 2: The figure shows total (PT) and reduced (PR) aminothiols in plasma
and total (ET) and reduced (ER) aminothiols in erythrocytes (RBC) before
(ND) and after 1-month treatment (ND t1) with GSH in patients affected by
Neurodegenerative Diseases (ND). Values are expressed as μM/L.
Cystine=CYS, Cysteine and Glycine=CYSGLY, Homocysteine=HCY,
Glutathione =GSH.
*=P‹0.05 with respect to ND.
CYS PT, ET, and ER levels increased significantly after GSH treatment.
CYSGLY PT levels increased significantly, and CYSGLY ET levels dropped significantly after GSH treatment.
HCY levels did not vary significantly after 1-month GSH treatment.
GSH PT and PR levels did not vary significantly after 1-month GSH treatment, whereas GSH ET and ER levels increased significantly after 1-month GSH treatment.
Figure 3 shows the percentage change from baseline of aminothiol levels after GSH treatment in ND patients. Percentage increases of CYS ET and ER and GSH ET and ER are important.

Figure 3: The figure shows the percentage modifications of total (PT),
reduced (PR) aminothiols in plasma and total (ET), and reduced (ER)
aminothiols in erythrocytes (RBC) following 1-month treatment with GSH in
patients affected by Neurodegenerative Diseases (ND).
Cystine=CYS, cysteine and glycine=CYSGLY, homocysteine=HCY,
glutathione =GSH.
The results obtained after three months of daily GSH treatment did not significantly vary compared with data obtained after 1-month daily GSH treatment (data not shown).
Discussion
Imbalance in oxidant production and antioxidant mechanisms contributes to neurodegeneration. Under physiological conditions, the regulation induced by antioxidant mechanisms such as GSH, as well as some transcriptional pathways, maintains low ROS/ RNS concentrations in neurons [2]. These low antioxidant levels help promote normal cell signaling, and might be neuroprotective. Under pathological conditions, such as in ND diseases, regulation of these antioxidant mechanisms decreases, with an increase in oxidant production that creates high ROS/RNS levels that can cause cell damage. GSH is the most abundant endogenous antioxidant nonprotein thiol in cells, and is a critical regulator of oxidative stress; it is able to detoxify drugs, protect macromolecules from oxidative damage, and maintain immune functions. GSH is synthesized from CYS, glutamic acid and Glycine (GLY) [20]. At a clinical level, disorders in GSH metabolism are more common than inborn GSHrelated enzyme errors in some ND, resulting in GSH depletion/ oxidative stress in the central nervous system [21]. It is plausible to say that GSH depletion might precede neurodegeneration.
Disorders of GSH metabolism in the brain in some ND have already been reported. MR spectroscopic imaging of GSH in white and gray matter at 7T has been studied: this non-invasive in vivo measurement shows a significant reduction of GSH in gray matter in MS patients compared to controls [22]. Moreover, low levels of GSH in the brain of secondary progressive MS patients measured using 1H MR chemical shift imaging at 3T have been reported [23]. We have already seen how patients affected by ND display low levels of GSH, which increase after antioxidant treatment [24]. GSH enhancement represents a potentially important approach in the treatment and prevention of disorders associated with GSH depletion. It was recently shown that oral supplementation with liposomal GSH elevates body stores of GSH and markers of immune functions [25].
In the present study, we evaluated the effects of GSH supplements on improving GSH levels in patients affected by ND or FM. GSH was used at a dose of 250 mg/day, with the aim to avoid the pro-oxidant effect of antioxidant chronic consumption [26]. Interestingly, a greater increase of GSH was seen after 500 mg/day than after 1000 mg/day GSH [25]. Additionally, in a previous study examining body stores of GSH following oral GSH supplementation, doses of 250 mg and 1000 mg GSH were used, showing that GSH levels increased analogously at both doses [27]. Characterization of the biochemical profile of different forms of aminothiol (reduced and total forms) might be relevant as a response to stimuli involving the redox state both in plasma and in RBC. The previously described new method was applied to assess the relationship between different types of thiol [Cystine (CYS), Cysteine and Glycine (CYSGLY), Homocysteine (HCY), and Glutathione (GSH)] in human plasma and in RBC under both redox homeostasis and oxidative stress [19]. Much evidence suggests that increased oxidative damage and decreased antioxidant function might be implicated in neurodegeneration in AD and PD [28,29]. The role of oxidative damage in the pathogenesis of AD and vascular dementia has also been proposed [30]. GSH is one of the most important agents in the endogenous antioxidant defense system; it can act as a cofactor of glutathione peroxidase which catalyzes the reduction of hydroperoxides. GSSH is a product of this reaction and is recycled into GSH by glutathione reductase. It has been shown that fish-oil supplements can increase glutathione reductase in MS patients [31]. Previous results demonstrated how daily consumption of GSH supplements was able to increase body compartment stores of GSH [27]. Moreover, the antioxidant and anti-inflammatory properties of the compound nerolidol have been shown to exert neuroprotective effects against neuroinflammation and oxidative stress induced by rotenone in male Wistar rats by increasing GSH levels [32]. Previously, the use of N-Actyl-Cysteine (NAC) in PD patients was able to exert protective effects on dopamine neurons [33]. Analogously, a single intravenous injection of NAC improves GSH levels in the human brain [34].
In the present study, some important differences regarding aminothiol content in plasma and/or in RBC were noted between controls and ND patients. CYS levels were significantly lower in PT and significantly higher in ET and ND patients compared to controls. Similar modifications were seen in CYSGLY contents. Since HCY levels were significantly higher in PT and ET of ND patients than in controls, our results suggest impairment of the HCY metabolism. In fact, the presence of higher levels of both CYS and CYSGLY was unable to give rise to an increase of GSH which was significantly lower in PT, PR, ET, and ER of ND patients than in controls. It is possible that the transsulfuration pathway (rather than the remethylation pathway) of HCY is impaired in ND patients, because an increased availability of CYS and CYSGLY does not seem to improve GSH levels. Treatment of ND patients with orally administered GSH significantly increases CYS levels both in PT and ET/ER. On the contrary, CYSGLY is improved by GSH treatment in PT, but is reduced in ET, suggesting its utilization for GSH synthesis. However, due to unaltered levels of HCY in ND GSH-treated patients, the significant increase of GSH in ET and ER seems due to GSH treatment only and not to stimulation of the HCY transsulfuration pathway. The percentage increase in GSH RBC levels in ND-treated patients shows the usefulness of our treatment in GSH-depleted patients. The recent demonstration that hippocampal neurons require a large pool of GSH to sustain dendrite integrity and cognitive function supports our results [35]. It is important to note that our MS-affected patients, whose GSH levels improved after the described treatment, decided not to use other drugs/integrators, and are presently performing EDTA chelation treatment associated with GSH assumption with continuous improvement of their symptoms.
Conclusion
We can say that ND patients display lower levels of GSH in plasma and in RBC compared to healthy patients. The treatment of ND patients with GSH restores GSH levels in RBC.
References
- Finkel T. Signal transduction by reactive oxygen species. The Journal of cell biology. 2011.
- Akhtar MW, Sunico CR, Nakamura T, Lipton SA. Redox regulation of protein function via cysteine S nitrosylation and its relevance to neurodegenerative diseases. International Journal of Cell Biology. 2012.
- Fernandez-Fernandez S, Almeida A, Bolaños JP. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochemical Journal. 2012.
- Dringen R. Oxidative and Antioxidative Potential of Brain Microglial Cells. Antioxidants & Redox Signaling. 2005.
- Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. In: Journal of Neuroscience Research. 2005.
- Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Molecular Aspects of Medicine. 2009.
- Pehar M, Ball LE, Sharma DR, Harlan BA, Comte-Walters S, Neely BA, et al. Changes in Protein Expression and Lysine Acetylation Induced by Decreased Glutathione Levels in Astrocytes. Molecular & Cellular Proteomics. 2016.
- Díaz-Hung ML, Yglesias-Rivera A, Hernández-Zimbrón LF, Orozco-Suárez S, Ruiz-Fuentes JL, Díaz-García A, et al. Transient glutathione depletion in the substantia nigra compacta is associated with neuroinflammation in rats. Neuroscience. 2016.
- Killoy KM, Harlan BA, Pehar M, Helke KL, Johnson JA, Vargas MR. Decreased glutathione levels cause overt motor neuron degeneration in hSOD1WToverexpressing mice. Experimental Neurology. 2018.
- Maher P. Potentiation of glutathione loss and nerve cell death by the transition metals iron and copper: Implications for age-related neurodegenerative diseases. Free Radical Biology and Medicine. 2018.
- Arús BA, Souza DG, Bellaver B, Souza DO, Gonçalves CA, Quincozes- Santos A, et al. Resveratrol modulates GSH system in C6 astroglial cells through heme oxygenase 1 pathway. Molecular and Cellular Biochemistry. 2017.
- Gokce EC, Kahveci R, Gokce A, Sargon MF, Kisa U, Aksoy N, et al. Curcumin Attenuates Inflammation, Oxidative Stress, and Ultrastructural Damage Induced by Spinal Cord Ischemia-Reperfusion Injury in Rats. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2016.
- Akkaya Ç, Yavuzer SS, Yavuzer H, Erkol G, Bozluolcay M, Dinçer Y. DNA damage, DNA susceptibility to oxidation and glutathione redox status in patients with Alzheimer’s disease treated with and without memantine. Journal of the Neurological Sciences. 2017.
- Mandal PK, Saharan S, Tripathi M, Murari G. Brain Glutathione Levels - A Novel Biomarker for Mild Cognitive Impairment and Alzheimer’s Disease. Biological Psychiatry. 2015.
- Chiang GC, Mao X, Kang G, Chang E, Pandya S, Vallabhajosula S, et al. Relationships among cortical glutathione levels, brain amyloidosis, and memory in healthy older adults investigated in vivo with1H-MRS and Pittsburgh compound-B PET. American Journal of Neuroradiology. 2017.
- Ehrhart J, Smith AJ, Kuzmin-Nichols N, Zesiewicz TA, Jahan I, Shytle RD, et al. Humoral factors in ALS patients during disease progression. J Neuroinflammation. 2015.
- Blasco H, Garcon G, Patin F, Veyrat-Durebex C, Boyer J, Devos D, et al. Panel of Oxidative Stress and Inflammatory Biomarkers in ALS: A Pilot Study. Can J Neurol Sci. 2017.
- Aaseth J, Alexander J, Bj�rklund G, Hestad K, Dusek P, Roos PM, et al. Treatment strategies in Alzheimer’s disease: a review with focus on selenium supplementation. BioMetals. 2016.
- Dellanoce C, Cozzi L, Zuddas S, Pratali L, Accinni R. Determination of different forms of aminothiols in red blood cells without washing erythrocytes. Biomedical Chromatography. 2014.
- Zhang H, Forman HJ. Glutathione synthesis and its role in redox signaling. Seminars in Cell and Developmental Biology. 2012.
- Aoyama K, Nakaki T. Impaired glutathione synthesis in neurodegeneration. International Journal of Molecular Sciences. 2013.
- Srinivasan R, Ratiney H, Hammond-Rosenbluth KE, Pelletier D, Nelson SJ. MR spectroscopic imaging of glutathione in the white and gray matter at 7 T with an application to multiple sclerosis. Magnetic Resonance Imaging. 2010.
- Choi I-Y, Lee S-P, Denney DR, Lynch SG. Lower levels of glutathione in the brains of secondary progressive multiple sclerosis patients measured by 1H magnetic resonance chemical shift imaging at 3T. Multiple sclerosis (Houndmills, Basingstoke, England). 2011.
- Fulgenzi A, Giuseppe R De, Bamonti F, Ferrero ME. Improvement of oxidative and metabolic parameters by cellfood administration in patients affected by neurodegenerative diseases on chelation treatment. BioMed Research International. 2014.
- Sinha R, Sinha I, Calcagnotto A, Trushin N, Haley JS, Schell TD, et al. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. European Journal of Clinical Nutrition. 2018.
- Pérez-Torres I, Guarner-Lans V, Rubio-Ruiz ME. Reductive stress in inflammation-associated diseases and the pro-oxidant effect of antioxidant agents. International Journal of Molecular Sciences. 2017.
- Richie JP, Nichenametla S, Neidig W, Calcagnotto A, Haley JS, Schell TD, et al. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. European Journal of Nutrition. 2014.
- Venkateshappa C, Harish G, Mahadevan A, Srinivas Bharath MM, Shankar SK. Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: Implications for neurodegeneration in Alzheimer’s disease. Neurochemical Research. 2012.
- Venkateshappa C, Harish G, Mythri RB, Mahadevan A, Srinivas Bharath MM, Shankar SK. Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: Implications for Parkinson’s disease. Neurochemical Research. 2012.
- Luca M, Luca A, Calandra C. The Role of Oxidative Damage in the Pathogenesis and Progression of Alzheimer’s Disease and Vascular Dementia. Oxidative Medicine and Cellular Longevity. 2015.
- Sorto-Gomez TE, Ortiz GG, Pacheco-Moises FP, Torres-Sanchez ED, Ramirez-Ramirez V, Macias-Islas MA, et al. Effect of fish oil on glutathione redox system in multiple sclerosis. Am J Neurodegener Dis. 2016.
- Javed H, Azimullah S, Abul Khair SB, Ojha S, Haque ME. Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neuroscience. 2016.
- Monti DA, Zabrecky G, Kremens D, Liang TW, Wintering NA, Cai J, et al. N-Acetyl cysteine may support dopamine neurons in Parkinson’s disease: Preliminary clinical and cell line data. PLoS ONE. 2016.
- Holmay MJ, Terpstra M, Coles LD, Mishra U, Ahlskog M, Öz G, et al. N-acetylcysteine boosts brain and blood glutathione in gaucher and Parkinson diseases. Clinical Neuropharmacology. 2013.
- Fernandez-Fernandez S, Bobo-Jimenez V, Requejo-Aguilar R, Gonzalez- Fernandez S, Resch M, Carabias-Carrasco M, et al. Hippocampal neurons require a large pool of glutathione to sustain dendrite integrity and cognitive function. Redox Biology. 2018.