
Mini Review
Austin J Clin Neurol 2021; 8(2): 1149.
Antepartum COVID-19 and Postpartum Autism
Steinman G* and Mankuta D
Department of Obstetrics & Gynecology, Hebrew University-Hadassah Hospital, Israel
*Corresponding author: Gary Steinman, Department of Obstetrics & Gynecology, Hebrew University-Hadassah Hospital, Ein Kerem, Jerusalem 12000, Israel; Email: dav4601@aol.com
Received: May 03, 2021; Accepted: May 19, 2021; Published: May 26, 2021
Abstract
A little over a year ago, a new viral disease appeared worldwide. Much like earlier pathologic RNA viruses, Covid-19 can cause distinctive harmful effects on pregnant women and their offspring. Because of the coexistent fever associated with a rise in pro-inflammatory interleukins in the most severe cases, there is a serious concern about the baby’s neurologic development. Although not yet observed in many Covid-19 pregnancies, it is anticipated particularly that the onset of autism in the child may be realized in a year or more postpartum. Prior studies have reported that exclusive breast-feeding which provides a good source of IGF1 for the baby may well reduce the incidence of autism in such cases.
Keywords: Cytokine storm; Interleukins; Respiratory distress; Proinflammatory; Alveoli
Introduction/Background
In the intrauterine development of a baby, the fetal stage is marked by varying levels of blood interleukins and IGF1 (insulin-like growth factor-1). If the baby is developing normally, without adverse influences in its environment, the trend over time is for serum interleukins to decrease and IGF1 to increase until delivery. On the other hand, if a maternal febrile cytokine storm occurs during the pregnancy, an overproduction of interleukins and reduction of IGF1 can take place. Such a phenomenon in the past has been associated with various bacterial and viral infectious diseases including cytomegalovirus, hemophagocytic lymphohistiocytosis, group A streptococcus, variola virus, influenza virus, SARS-CoV-1 (severe acute respiratory syndrome – a coronavirus), and avian H5N1 virus. Without fever, adverse manifestations are reduced [1-10].
Since 1918, orthomyxoviruses [single-stranded RNA viruses], such as influenza, have been the cause of epidemics and pandemics every few years. These viruses are spherical with distinctive surface spikes made of Hemagglutinin (HA) or Neuraminidase (NA) protein. Beginning with 2020, Covid-19, the newest member of this pathologic microbial group, has exhibited aspects of cytokine excesses in many cases. Much like the earlier pandemics of Spanish flu and H5N1, such a flare-up is characterized by fever, lethargy, and dyspnea. These zoonotic viruses are increasingly proliferative as the human population grows [3,6].
A consistent finding in patients with pulmonary complications is the overproduction of specific pro-inflammatory interleukins. Cytokines can elicit responses in cell proliferation and inflammatory reactions. Interleukin 1 (IL1), IL6, IL17, and TNF (tumor necrosis factor) in particular are central to the overt harmful features of acute viral diseases [7,11,12]. Especially with Covid-19, the most serious damage appears to be localized in the lung alveoli, where the viral spikes attach to ACE2 receptors. As the pathogenesis progresses, lung injury can lead to Acute Respiratory Distress Syndrome (ARDS) [6- 11,13].
Current therapy protocols for difficult cases of COVID-19 include drugs such as Tocilizumab (TCZ), a monoclonal antibody against IL6 receptor in patients with life-threatening cytokine storm [14-16]. In combination with methylprednisolone, this therapeutic agent is reported to successfully reduce cardiovascular collapse and major organ dysfunction in a number of severely ill coronavirus patients. Another relatively new agent, Allocetra, is effective in reprogramming macrophages and dendritic cells [17].Under normal postpartum conditions, the serum IGF1 level rises until the early teen years, after which it slowly decreases over time. In contrast, when a fetus had faced a fever-generating antepartum state such as a severe maternal coronavirus attack, the levels of serum IGF1 of the newborn are diminished and of pro-inflammatory Interleukins (e.g., IL6) are increased [18,19]. In a postmortem examination of human autistic brains, increased cytokines and pathologic signs of inflammation have been detected [1].
Maternal death often follows a failure to recover from immune paralysis and irreversible lung injury. As in similar cytokine enhancing events with other febrifacient viruses, one would anticipate an upsurge in the number of autistic children in the coming months or years with mothers who experienced febrile antepartum Covid-19 [12,20-23]. Antipyretics in gravidas have been somewhat successful in lowering the autism rate in viral illnesses [11].
Proposed Therapy
It has been reported with previous viral pandemics that gravidas suffering from microbe-induced fevers give birth to children who are more likely to exhibit autistic traits later. From various studies, this is apparently related to a deficiency of IGF1 and a persistence of enhanced IL6 in the baby after birth [5,7]. Such an IGF1 deficit reduces the rate of myelination and functional assembly of neoneuronal circuits in the baby’s brain (dysconnectivity) [24].
In laboratory fetal mice, increased serum IL6 is concomitant with low IGF levels in utero. In unaffected humans born at a mean gestational age of 27.8 weeks, for example, the average serum concentration of IGF1 is 46.6 ng/l, whereas the concentration of IGF1 is approximately double in full term neonates. In otherwise normal pregnancies, the serum IL6 at term is about a quarter of what it was in the middle of the gestation [25,26].
In pregnancies where the gravida had suffered a serious ARDS event before delivery, the newborns sustain IL6 levels at birth and beyond which are much higher than normal. Similarly, children with overt autism are typically found to have elevated serum IL17 [23,26,27]. In other words, autism is more likely to arise postpartum if the serum IGF1 level is depressed concurrently, as in severe COVID-19.
The hypothetical diagram (Figure 1) displays the typical fall in serum IL6 and the rise of IGF1 preceding the infected phase of a pregnancy, as well as the opposite trends following a Covid-19 infective/febrile event. Gross symptoms of autism in the baby usually become evident after the first year of postpartum life [23,26-28].
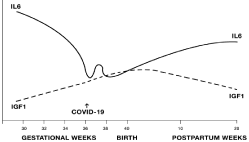
Figure 1: Relationship between fetal/neonatal serum IL6 and IGF1 before
and after a febrile Covid-19 attack [5,7,21,25,26,35-40].
An efficacious means for supplying IGF1 to the affected newborn currently is breast-feeding. Human breast milk typically contains larger concentrations of IGF1 than milk from other sources [29- 31]. In general, autism is less common in children who have been fed breast milk only for up to one full year initially. It has also been reported that the higher the serum IGF1 level of the gravida, the lower is her own risk of Covid-19 death [32]. The spontaneous lowering of IGF1 with age might explain why older people have an increasing chance of dying once COVID-19 is contracted [33].
Such observations would suggest that virally affected pregnant women could also be treated with supplemental IGF1, especially at the critical time of a harsh attack. Vertical transfer of COVID-19 from the mother to the unborn child is very uncommon [23]. It is now accepted practice in the U.S. to fortify very premature neonates with IGF1, leading to improved states in the first weeks after delivery [34].
As Covid-19 appeared around the beginning of 2020, it will be of interest to evaluate the veracity of this model once the babies of infected parturients reach an age of 1-3 years.
Conclusions
The past [41] and present reports are intended to provide an encompassing conceptual basis for elucidating the biochemical phenomena underlying the origin and prevention of autism. Prior research work in this subject has defined our understanding of what causes some, many, or all cases of autism. In this way, our current studies are sharpened as follows:
• Autism and IGF1 deficiency: IGF1 directly affects the rate at which oligodendrocytes promote myelination and neural circuitry development in the infantile CNS. Factors which reduce the production or availability of IGF1 could retard normal nerve programming in the fetus or neonate, leading to autism or schizophrenia.
• Breast-fed babies possess increased IGF1 and less frequent autism: The emergence of autism in young children appears to result from dysmyelination and dysconnectivity of brain neurons related to inadequate supply of IGF1 in the newborn. Breast-feeding for the first year following birth promotes the decreased incidence of autism [42,43].
• Prevention of later autism tendency in fetuses/neonates of febrile gravidas: Risk of autism is raised when the fetus is carried by a febrile infected mother with elevated IL1, IL6, IL17, and TNF proinflammatory interleukins. Few antiviral medications have sofar been tested rigorously to avoid fetal/neonatal side-effects, thereby limiting their use in pregnant cases. Immediate postpartum treatment with IGF1 supplementation is feasible (e.g., IV infusion) when umbilical cord blood testing indicates need.
Acknowledgement
The authors wish to thank Roberta Zuckerman for her helpful discussions about the basis and structure of this communication, as well as Aviva Adler, librarian, for her cooperative assistance in locating relevant references. No external funds were made available for completion of this study. Joseph Tocker expertly prepared Figure 1. The authors have no conflict of interest in this report.
References
- Vargas DL, Nascimbene D, Krishnan C, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of Neurology. 2005; 57: 67-81.
- Zimmerman, AW, Jyonouchi, H, Comi, AM, et al. Cerebrospinal fluid and serum markers in autism. Pediatric Neurology. 2005; 33: 195-201.
- Lathe R. Autism, Brain, and Environment. Kingley Publishers, London. 2006; 156-162.
- Vanhalla R, Turpeinen U, Riikonen R. Low levels of insulin-like growth factor-1 in cerebrospinal fluid in children with autism. Developmental Medicine & Child Neurology. 2001; 43: 614-616.
- Patterson PH. Maternal infection and immune involvement in autism. Trends in Molecular Medicine 2001; 17: 389-394.
- Lostroh P. Molecular and cellular biology of viruses. CRC Press, Boca Raton, Fl. 2019.
- Tisoncik JR, Kort N, Simmons, CP, et al. Into the eye of the cytokine storm. Microbiology Molecular Biology Reviews. 2012; 76: 16-32.
- Croen LA, Qian Y, Ashwood P, et al. Infection and fever in pregnancy and autism spectrum disorders: Findings from the study to explore early development. Autism Research. 2019; 12: 1551-1561.
- Hornig, M, Bresnahan, MA, Che, SX, et al. Prenatal fever and autism risk. Molecular Psychiatry. 2018; 23: 759-766.
- Mahic M, Che X, Susser E, et al. Epiemiological and serological investigation into the role of gestational maternal influenza virus infection and autism spectrum disorders. Msphere. 2017.
- Zerbo A, Iosif A, Walker C. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Result from the CHARGE Study. Journal of Autism and Developmental Disorders. 2013; 43: 25-33.
- Tioleco N, Silberman AE, Stratigos K, et al. Prenatal maternal infection and risk for autism in offspring: A meta-analysis. Autism Research. 2021; 3: 1-21.
- Barr SM, Johnson MA, Janossy G. Cytopathology or immunopathology? The puzzle of cytomegalovirus pneumonitis revisited. Bone Marrow Transplant, 2000; 26: 591-597.
- Raucci F, Mansour AA, Casillo GM, et al. Interleukine-17A (IL-17A), a key molecule of innate and adaptive immunity and its potential involvement in Covid-19-related thrombotic and vascular mechanisms. Autoimmunity Reviews. 2020; 19: 102572.
- Lu P, Liu Y, Qui A, et al. Tocilizumab treatment of COVID-19: A single center experience. Journal of Medical Virology. 2020.
- Rossotti R, Travi G, Ughi N, et al. Safety and efficacy of anti-IL-receptor tocilizumab use in severe and critical patients affected by coronoavirus disease: a comparative analysis. Journal of Infections. 2019; 81: e11-17.
- Trahtemberg J, Mevorach D. Apoptotic cells induced signaling homeostasis in macrophages and dendritic cells. Frontiers of Immunology. 2017; 8: 1356- 1362.
- Cartmell T, Poole S, Turnbull AV, et al. Circulating interleukin-6 mediates the febrile response to localized inflammation in rats. Journal of Physiology. 2000; 526: 653-661.
- Choi C Yim YS, Wong H, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016; 351: 933-939.
- Kim JS, Lee JY, Yang JW, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021; 11: 316-329.
- Leija-Martinez JJ, Huang F, Del-Rio-Navarro BE, et al. IL-17A and TNF-α as potential biomarkers for acute respiratory distress syndrome and mortality in patients with obesity and COVID-19. Medical Hypotheses. 2020; 144: 109935.
- Martins-Filho PR. COVID-19 during pregnancy: Potential risk for neurodevelopmental disorders in neonates? European Journal of Obstetrics & Gynecology and Reproductive Biolology. 2020; 250: 255-256.
- Chen LYC, Hoiland RL. Stilas S. et al. Confronting the controversy: Interleukin and Covid-19 cytokine storm syndrome. European Respiration Journal. 2020.
- Steinman, G, Mankuta, D. COVID-19 and autism–Part 2. Integrated Gynecology and Obstetrics Journal 2021; 3: 1-3.
- Bidlingmaier M, Friedrich N, Emeny RT, et al. Reference intervals for Insulin- Like Growth Factor-1 (IGF-1) from birth to senescence. Journal of Clinical Endocrinology and Metabolism, 2014; 99: 1712-1721.
- Cirillo G, Lazzerone P, Sartori D, et al. Inflammatory diseases and growth: Effects on the GH-IGF axis and on growth plate. International Journal of Molecular Sciience. 2017; 18: 1878-1887.
- Wrigley S, Arafa D, Tropea D. Insulin-like growth-1: At the crossroads of brain development and aging. Frontiers of Cell Neuroscience. 2017; 11: 1-15.
- Ai-Ayadhi, LY, Mostafa, GA. Elevated levels of interleukin-17A in children with autism. Journal of Neuroinflammation. 2012; 9: 158-164.
- Shamsedine L, Mailhac A, Badaoui B, et al. Breastfeeding association with autism spectrum disorders: A case-control study from Lebanon. Research in Autism Spectrum Disorders. 2020; 78: 101651.
- Shafai T, Mustaafa M, Hild T, et al. The association of early weaning and formula feeding with autism spectrum disorders. Breastfeeding Medicine. 2014; 9: 275-276.
- Schultz ST, Klonoff-Cohen HS, Wingard DL, et al. Breastfeeding, infant formula supplementation and autistic disorder: the results of a parent survey Int Breastfeeding Journal. 2001.
- Fan S, Yin C, Wang J, et al. Pre-diagnostic circulating concentration of insulin-like growth factor-1 and risk of Covid-19 mortality: results from UK Biobank. European Journal of Epidemiology. 2021.
- Undurraga EA, Chowell G, Mizumoto K. COVID-19 case fatality risk age and gender in a high testing setting in Latin America: Chile, March–August 2020. Infectious Diseases in Poverty. 2021; 10: 11.
- Hellstrom A, Ley D, Hansen-Pupp I, et al. Role of insulinlike growth factor 1 in fetal development and in the early postnatal life of premature infants. American Journal of Perinatology. 2016; 33: 1067-1071.
- Steinman G. Prenatal identification of autism propensity. Medical Hypotheses. 2019; 122: 210-211.
- Sukhanov S, Higashi Y, Shai S-Y. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in apoE-deficient mice. Arteriosclerosis Thrombosis Vascular Biology. 2007.
- Shmad I, Zaldivar F, Iwanaga K, et al. Inflammatory and growth mediators in growing preterm infants. Journal Pediatrics Endocrinology & Metabolism. 2007; 20: 387-396.
- Street ME, Sheinin P, Fieni S, et al. Changes in interleukin-6 and IGF system and their relationships in placenta and cord blood in newborns with fetal growth restriction compared with controls. European Journal Endocrinology. 2006; 155: 567-574.
- Rudolph MD, Graham AM, Feczko G, et al. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Natural Neuroscience. 2018; 21: 765-772.
- Jiang H, Lian-lian X, Shao L, et al. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain Behavior Immunity. 2016; 58: 165-172.
- Steinman G. The putative etiology and prevention of autism In Progress in Molecular Biology and Translational Science–Autism; chapter 1. M. Ilieva and W.K. Lau, editors. Elsevier/Academic Press, Cambridge, MA, USA. 2020: 173.
- Kuemmerle, JF. Insulin-like growth factors in the gastrointestinal tract and liver. Endocrinology and Metabolism Clinics of North America. 2012; 41: 409- 423.
- DeBoer MD, Scharf, RJ, Leite, AM, Ferrer A, Havt A and Pinkerton R, et al. System inflammation, growth factors, and linear growth in the setting of infection and malnutrition. Nutrition. 2016; 33: 248-253.