Review Article
Austin J Clin Ophthalmol. 2014;1(3): 1012.
The P2X7 Receptor in AMD
Dongli Yang1* and Jun Chen2
1Departments of Ophthalmology and Visual Sciences, University of Michigan, USA
2Departments of Internal Medicine, University of Michigan, USA
*Corresponding author: Dongli Yang, Department of Ophthalmology and Visual Sciences, University of Michigan, 344 Kellogg Eye Center, , 1000 Wall Street, Ann Arbor, MI 48105-5714, USA
Received: February 20, 2014; Accepted: February 24, 2014; Published: March 03, 2014
Abstract
Age–related macular degeneration (AMD) is the leading cause of blindness among people over the age of 50 worldwide. However, its exact causes and the underlying mechanisms remain largely unknown. The P2X7 receptor (P2X7R) is an ATP–gated cationic channel expressed in retina. Recent advances have highlighted the P2X7R–mediated pathophysiological processes in the development of AMD. This review will discuss the current literature regarding P2X7R in the RPE physiological and pathophysiological processes, and assess its potential impact with respect to AMD.
Keywords: AMD; apoptosis; P2X7; RPE.
Abbreviations
AMD: Age–related Macular Degeneration; ASC: Apoptosisassociated Speck–like protein containing a Caspase recruitment domain; ATP: Adenosine Triphosphate; BBG: Brilliant Blue G; BzATP: 2’3’–O–(4–benzoylbenzoyl)–ATP; CNV: Choroidal Neovascularization; IL–8: Interleukin 8; KN–62: 4– [(2S)–2– [(5–isoquinolinylsulfonyl) methylamino] –3 – oxo – 3 – ( 4 – phenyl – 1 –piperazinyl) propyl] phenyl isoquinolinesulfonic acid ester; MCP– 1: Monocyte Chemoattractant Protein–1; NF–?B: Nuclear Factor ?B; NLRP3: Nucleotide–binding domain and Leucine–Rich repeat containing family, Pyrin domain containing 3; oATP: Oxidized ATP; POSs: Photoreceptor Outer Segments; PPADS: Pyridoxal–Phosphate– 6–Azophenyl–,2’,4’–Disulphonic Acid; P2X7R: P2X7 Receptor; ROS: Reactive Oxygen Species; RPE: Retinal Pigmented Epithelium; Snps: Single Nucleotide Polymorphisms; VEGF: Vascular Endothelial Growth Factor
Introduction
Age–related macular degeneration (AMD) is the leading cause of blindness among people over the age of 50.It is a worldwide epidemic. In a cross–sectional study with 4 racial/ethnic groups aged 45–84 years, early AMD and late AMD were present in 4.0% and 0.5% of the cohort, respectively, varying from 2.4% and 0.2% in blacks, 3.8% and 0% in Hispanics, and 3.8% and 1.1% in Chinese to 6.0% and 0.5% in whites, respectively [1]. In a large retrospective longitudinal cohort study, among 2 259 061 individuals (whites, blacks, Latinos, and Asians) aged ≥40 years, 113 234 (5.0%) were diagnosed with non exudative and 17 181 (0.76%) with exudative AMD [2]. In a Chinese population aged ≥40 years, the prevalence of early, late, and neovascular AMD was 5.2%, 0.2% and 0.1%, respectively, and the incidence of per subject was 4.2%, 0.1%, and 0.1%, respectively [3]. The prevalence of AMD rises steeply with age. In a study with three racially similar populations of 14 752 individuals from North America, Europe, and Australia, AMD affects nearly 0.2% of the population aged 55 to 64 years, and 13% of the population older than 85 years [4].The estimated prevalence of late AMD was 0.08% at age 50, 0.33% at age 60, 1.38% at age 70, 5.60% at age 80, and 20.10% at age 90, respectively [5]. As global population ages, the burden on healthcare systems worldwide related to treating this chronic disease will be overwhelming.
AMD is a progressive degeneration of the macula, the portion of the retina used for central vision. Retina consists of the inner neural retina, and the outer retinal pigment epithelial (RPE) layer. The RPE layer sits on Bruch’s membrane, forms the outer blood–retina barrier, separates the neural retina from its choroidal blood supply, and maintains a physiological environment for photoreceptor function. This RPE monolayer is a main target in the development of AMD. The earliest stage of AMD is characterized by an accumulation of extracellular lipid– and protein–containing deposits, termed drusen, between the RPE and Bruch’s membrane. As AMD progresses, it can develop into two distinct forms of late or advanced AMD: “dry” AMD (geographic atrophy) and “wet” AMD (neovascular AMD). The “dry” AMD is the most common form (90%), characterized by the slow loss or blurring of central vision in spots due to significant RPE/ neuro retinal atrophy. The “wet” AMD is less common (10%), more severe, and may progress rapidly and cause the most severe vision loss because of proliferation and invasion of abnormal choroidal (or occasionally retinal) blood vessels and fluid leakage into the retina [6–9].
The exact causes and the underlying pathogenic mechanisms for AMD remain largely unknown, but numerous studies have established advanced age, smoking, and genetic predisposition as key risk factors. Other risk factors include low dietary intake of antioxidants, dietary fat intake, gender, race, ethnicity, cardiovascular disease, high blood pressure, cholesterol levels, estrogen levels, and light exposure [9,10]. The possible mechanisms for AMD include genetic, epigenetic and environmental factors related to RPE senescence, alterations in the complement pathway, increased inflammation, changes in the balance of growth factors, excessive lipofuscin accumulation, mitochondrial defects, and oxidative stress [6,8].Currently, there is neither a cure nor means of prevention for AMD [8,9]. Many completed and ongoing immune–based clinical trials for AMD have been ineffective [7]. There is, therefore, a critical need to identify new mechanisms for AMD, in order to develop unique preventive and therapeutic strategies for this age–related blinding disease.
The purinergic receptor P2X, ligand–gated ion channel, 7 (P2X7R; also known as P2RX7, P2X7 receptor, P2X7, P2X7 or P2Z) is an ATPgated cationic channel expressed by a variety of cell types including hematopoietic, epithelial, and neuronal cells [11,19]. The P2X7R is involved in oxidative stress, cell death and inflammatory processes, all of which have been linked to AMD.
This review will discuss the most recent advances in the P2X7R, focusing on the P2X7R in the RPE and its implications in AMD pathogenesis.
The P2X7 Receptor
Virtually all types of cells express plasma membrane receptors for extracellular nucleotides termed P2 receptors that are further categorized into P2X receptors and P2Y receptors [20]. So far, fifteen P2 receptors have been identified, including seven P2X receptor subunits (P2X1–7), and eight P2Y receptor subtypes (P2Y1,2,4,6,11,12,13,14). P2X receptors are ligand–gated, nonselective cation channels, ranging from 379 to 595 amino acids in length. Each subunit of P2X receptors is composed of two transmembrane domains (TM1 and TM2), a large extracellular loop, and intracellular N– and C–termini. P2X receptor subunits co–assemble to form functional homotrimeric or heterotrimic forms depending on tissue–specific expression and receptor subunits. P2X receptors are activated by extracellular ATP. Activation of P2X receptors causes influx of Ca2+ and Na2+ and efflux of K+.P2Y receptors are classical heterotrimeric G protein–coupled receptors featuring an extracellular N–terminus, seven transmembrane domains, and an intracellular C–terminus. P2Y receptors are activated by ATP, ADP, UTP and UDP.
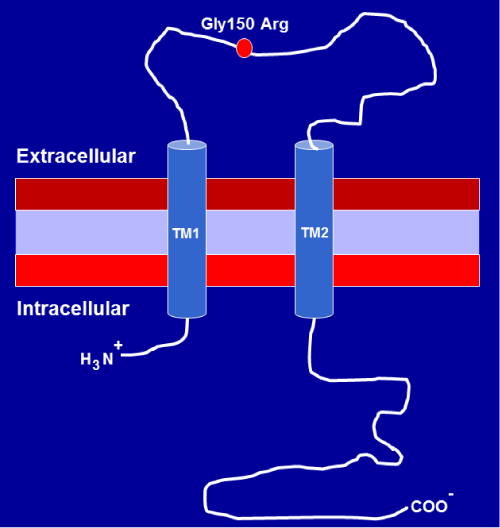
Among seven P2X receptors, the P2X7R is unique in terms of both its structure and function. The human P2X7R gene is localized within a 55–kb region of chromosome 12q24, is highly polymorphic and has 13 exons that encode a 595 amino acid polypeptide [21]. The C–terminus (244 aa) of P2X7R is 120–200 amino acids longer than that of the other P2X receptors, and harbors multiple potential protein and lipid interaction motifs, which was thought to be pivotal in regulating its function [22]. Increasing evidence suggests that the C–terminusis critical for post–translational modification, cellular localization, oligomerization, protein–protein interactions and signaling pathway activation [23,24]. A schematic structure of the P2X7R is shown in (Figure 1).
Figure 1a :Diagrammatic representation showing the membrane topology of P2X7receptor subunit. First and second transmembrane domains are labeled TM1 and TM2. The position of a single amino acid substitution from glycine to arginine at residue 150 (Gly150Arg) as a result of P2X7 receptor 474G>A (rs28360447) gene polymorphism is shown on the diagram.
The position of a single amino acid substitution from glycine to arginine at residue 150 (Gly150Arg) as a result of P2X7 receptor 474G>A (rs28360447) gene polymorphism is shown on the diagram.
Stimulation of P2X7R with low ATP doses, reversibly opens a membrane channel permeable to small cations (Na+, Ca2+, K+), while prolonged exposure with higher ATP doses or repeated stimulation with sequential ATP leads to the formation of a nonselective pore permeable to molecular mass up to 900 Da, which can result in cell death by either apoptosis or necrosis [11,12,25]. Aging has been associated with increased expression of the pro–inflammatory cytokines and chemokines [26–31]. Inflammation and oxidative stress are hallmarks of aging, and have been linked to a wide spectrum of age–related disorders, including Alzheimer’s disease and AMD. Evidence indicates that the P2X7R is involved in aging, oxidative stress, inflammation, as well as age–related disorders, such as such as Alzheimer’s disease [32,34], and AMD [17,35–37].
P2X7R Polymorphisms
The human P2X7R gene contains more than 260 single nucleotide polymorphisms (SNPs). Among them, functional polymorphisms that increase (gain–of–function) or decrease (loss–of–function) function of the P2X7R are characterized, and a few polymorphisms have been associated with diseases. Non–synonymous SNPs and the corresponding literature are listed in Table 1.
dbSNP ID
Base change
Exon
Amino acid change
Function compared to WT or Disease Association
Reference
rs35933842
151+1g → t
1
Null allele
associated with increased fracture risk and reduced BMD in women
[92]
[100]
rs1752809
253T → C
2
Val76 to Ala
Partial inhibition
[45]
rs28360447
474G → A
5
Gly150 to Arg
loss-of-function-disrupted protein folding, no pore formation
associated with increased susceptibility to AMD
associated with decreased hip BMD values
associated with decreased total hip BMD in women and men combined
[44,45]
[35]
[102]
[100]
rs208294
489C → T
5
His155 to Tyr
gain-of-function
[45,93, 94]
rs7958311
835G → A
8
His270 to Arg
gain-of-function
[45]
rs7958316
853G → A
8
Arg276 to His
loss-of-function
[45]
rs28360457
946G → A
9
Arg307 to Gln
loss-of-function
associated with the rate of bone loss in post-menopausal women
[95]
[101]
rs1718119
1068G → A
11
Ala348 to Thr
gain-of-function
enhanced interleukin-1ß secretion
associated with a lower vertebral fracture incidence 10 years after menopause in post-menopausal women
associated with increased BMD values at the lumbar spine
associated with reduced fracture risk and increased BMD
[94,96]
[45]
[101]
[102]
[100]
rs2230911
1096C → G
11
Thr357 to Ser
loss-of-function
[97]
rs2230912
1405A → G
13
Gln460 to Arg
small reduction, no major effect or gain-of-function
a significantly decrease in risk of a lower BMD T-score value
associated with increased total hip BMD in women
[44,45,93, 94]
[102]
[100]
rs3751143
1513A → C
13
Glu496 to Ala
loss-of-function; surface expression not affected
associated with protection against bone loss in post-menopausal women
associated with a decreased risk of IHD in smokers as well as decreased risk of IS
significantly associated with increased susceptibility to tuberculosis
associated with decreased hip BMD values
decreased lumbar spine BMD in women and decreased total hip BMD in men
[98]
[101]
[103]
[104]
[102]
[100]
rs1653624
1729T → A
13
Ile568 to Asn
loss-of-function
had increased bone loss
[98,99]
[101]
Table 1: Single Nucleotide Polymorphisms (SNPs) in P2X7Receptor.
By comparing amino acid sequences, it was found that P2X7R is more homologous to P2X4R (˜40%) than are the other P2X receptor subunits. Given their location adjacent to each other on human chromosome 12, and their overlap in tissue distribution [38,39], efforts have been made to identify if there is a physical and functional interaction between the two receptors. Several studies have found that P2X4R and P2X7R are co–expressed in immune cells and epithelial cells, and heteromerization can change both the functional and pharmacological properties of P2X receptors [40–43].
A loss–of–function polymorphism has been identified in human P2X4R Tyr315Cys (rs28360472) which is associated with increased susceptibility to AMD [35]. P2X7R 474G>A (Gly150Arg) (rs28360447) gene polymorphism leads to a single amino acid substitution from glycine to arginine at residue 150 (Figure 1), producing loss–of–function channel [44,45]. Haplotype analysis showed that the P2X4R 315–Cys minor allele was co–inherited with P2X7R 150–Arg 4–fold more often in patients with AMD than in normal control subjects [35]. Among 17 patients with AMD inheriting the haplotype of P2X4R315–Cys plus P2X7R 150–Arg, 14 were female [35]. Infiltrating macrophages within the choroid and microglia within a monkey neural retina were found to co–express P2X4R and P2X7R [35]. In mouse bone marrow–derived dendritic cells, it was found that expression of P2X4R is required for P2X7R–dependent IL–1β and IL– 18 release [46]. Based on Ca2+ influx triggered by ATP and BzATP was insensitive to suramin, we suggested that in addition to P2X7R, P2X4R could contribute to ATP– and BzATP–induced Ca2+ influx in the RPE [17]. Future studies are needed to test this hypothesis.
The P2X7R is a new scavenger receptor for bacteria and apoptotic cells in the absence of serum and extracellular ATP [47], suggesting the unstimulated P2X7R could have a beneficial role under physiological conditions. Whether the P2X7R is a new scavenger receptor in the RPE needs to be investigated. This is important, because one of the RPE’s essential functions is phagocytosis, removing photoreceptor outer segments (POSs) to maintain neural retina health [48]. Both human and mouse RPE express the P2X7R [17–19,49]. Moreover, P2X7R protein is expressed on both apical and basolateral membranes of mouse RPE monolayer in situ [19]. Compared to human macrophages, ARPE–19 cells (a human RPE cell line) are more efficient in clearing anoikic and UV–induced apoptotic cells [50], suggesting the importance of the RPE in clearance of dying cells and extracellular debris. The P2X7R expressed on apical membrane could participate in phagocytosis of POSs, while the P2X7R expressed on basolateral membranes could be important for clearance of apoptotic cells and against invading bacteria.
The P2X7R in the RPE
The P2X7R is expressed in epithelial cells [13–19], and up regulated by lipopolysaccharide and pro–inflammatory cytokines [18,51,52]. In the RPE, the expression of the P2X7R is also up regulated by aging [18]. Under normal physiological conditions, P2X7Ractivity is kept at a low level by the concentration of extracellular divalent cations [53,54] as well as by low micromolar range of extracellular ATP. Extracellular divalent cations appear to alter the affinity of ATP binding in an allosteric manner [55]. Low micromolar range of extracellular ATP is not favored for P2X7R activation, as the P2X7R is the least sensitive member of the P2X receptor family to activation by ATP with EC50value of 0.1 to 1 mM compared with P2X1– 6receptors whose EC50value is 1 to 10 µM [17,56–58]. This could avoid unnecessary cell permeability and pore formation [59]. Under stress conditions, both RPE cells and neural retina are capable of releasing ATP that can act on P2X receptors in RPE cells and⁄or photoreceptors in an autocrine or a paracrine manner [17,36]. Among seven P2X receptors, P2X7R mRNA and protein have been identified in the RPE by three independent research groups [17–19,49]. We detected not only P2X7R protein, but also P2X7R mRNA in human RPE cells [17]. Guha also detected both P2X7R mRNA and protein in mouse RPE cells, with the P2X7R protein expressed on both apical and basolateral membranes of mouse RPE monolayer in situ. Compared to wild–type mice, P2X7R mRNA in fresh RPE⁄choroid tissue was increased in ABCA4–/– mice, a mouse model of Stargardt’s retinal degeneration [19]. We also found that aging and inflammation up regulated the expression of P2X7R mRNA and protein in the RPE [18]. However, Gu [35], reported that P2X7R protein was not detected in retinal sections from an adult monkey (Macaca fascicularis) eye by using immunofluorescent labeling method. P2X7R mRNA was not reported in their study. The discrepancies between the report by Gu et al. [35] and other three independent research groups [17,19,49] could be explained by species difference. In addition, different sources of commercially available antibodies [60,61] used could play a role. Therefore, caution should be taken when interpreting P2X7R protein expression data obtained by immunocyto chemistry.
The P2X7R is functional in the RPE.ATP is an endogenous P2X7R agonist, while 2’,3’–O–(4–benzoylbenzoyl)–ATP (BzATP) is a synthetic, selective P2X7R agonist [62]. Both ATP and BzATP induce RPE apoptosis after 6 hr or 24 hr stimulation, and increase intracellular Ca2+ via extracellular Ca2+ influx rapidly in primary human RPE [17]. BzATP also raises intracellular Ca2+ in ARPE– 19cells [19]. However, ARPE–19 cells exposed to BzATP (50 or 100 µM) for 60 minutes did not release lactose dehydrogenase into the extracellular media [19], indicating that short exposure did not kill ARPE–19 cells. Both ATP and BzATP increased YO–PRO–1 (629 Da) dye uptake in a human RPE cell line, ARPE–19 cells [49]. BzATP also triggered a rapid and reversible elevation of Ca2+ in freshly isolated mouse RPE cells [19]. These data indicate that activation of P2X7R by ATP or BzATP not only opens a membrane channel permeable to Ca2+, but also leads to the formation of membrane pore permeable to YO–PRO–1, and cell death in RPE cells. Functional P2X7R in the RPE was further validated in the RPE by using P2X7R antagonists, oxidized ATP (oATP), brilliant blue G (BBG), and 1–[N,O–Bis(5– isoquinolinesulfonyl)–N–methyl–L–tyrosyl]–4–phenylpiperazine (KN– 62). Oxidized ATP significantly inhibited ATP– or BzATP–induced Ca2+ influx and apoptosis by the RPE. BzATP–induced RPE apoptosis was blocked or significantly inhibited by P2X7R antagonists BBG, KN–62, and oATP [17]. Reduction or removal of extracellular Ca2+ or the buffering of intracellular Ca2+ with BAPTA–AM significantly inhibited or blocked ATP–induced apoptosis [17]. These findings suggest that the P2X7R contributes to ATP– and BzATP–induced Ca2+ signaling and apoptosis in the RPE. Therefore, abnormal Ca2+ homeostasis and membrane pore formation through the activation of P2X7R could cause the dysfunction and apoptosis of RPE that underlie AMD.
One of major functions of the RPE is to degrade phagocytosed POSs. Guha et al. [19] demonstrate that stimulation of P2X7Rby 100 µMBzAT Palkalinizes lysosomes in ARPE–19 cells. Although P2X7R antagonistA438079 [63] had greater potency in blocking BzATP–induced intracellular Ca2+ increase in recombinant mouse, rat or human P2X7R–expressed human astrocytoma 1321N1 cells, compared with BBG [64], the potency of A438079 to inhibit BzATPinduced increase in lysosomal pH in ARPE–19 cells seemed lower, compared with BBG. A438079 at 10 µM and BBG at 1 µM suppressed BzATP–induced increase in lysosomal pH to a similar extent [19]. This BzATP–induced lysosomal alkalinization was dependent on extracellular Ca2+, because BzATP was unable to increase lysosomal pH in the absence of extracellular Ca2+ [19], indicating P2X7R plays a role in lysosomal alkalinization. Lysosomal enzymes function optimally at low pH. Thus, lysosomal alkalinization could impair lysosomal function. Indeed, they found that blockage of the P2X7R by BBG was able to reduce lipid oxidation and lipofuscin–like autofluorescence induced by POSs plus lysosomotropic agent chloroquine [19]. Cathepsin D is a major proteolytic enzyme participating in the lysosomal digestion of phagocytosed POSs [65,66]. BODIPY FLpepstatin A selectively binds to cathepsin D at pH 4.5 [67]. Lysosomal alkalinization induced by BzATP reduced BODIPY FL–pepstatin A binding to catheps in D [19], indicating the ability of catheps in D to digest proteins is compromised. Furthermore, stimulation of ARPE–19 cells with BzATP increased the ratio of LC3BII/LC3BI (autophagy markers), and decreased the level of p62 (autophagy adaptor protein), supporting impairment of autophagic flux [19]. Recently, [68] discovered LC3–associated phagocytosis in which the interplay between phagocytosis and autophagy within the RPE is required for degradation of POSs and the maintenance of retinoid levels to support optimal vision. Given that the P2X7R is involved in both autophagy [19,69] and phagocytosis [47], we anticipate that the P2X7R could play a role in this LC3–associated phagocytosis.
The P2X7R in AMD
Oxidative stress, inflammation, and cell death are implicated in AMD [8,70–73]. As activation of P2X7R induces Ca2+–dependent apoptosis and lysosomal alkalinization in the RPE [17,19], we propose that abnormal Ca2+ homeostasis, oxidative stress, inflammation, and cell death are important factors for the development of AMD (Figure 2). The RPE maintains a healthy environment for normal photoreceptor function. In dry or atrophic AMD, it appears that the RPE dies first, leading to dysfunction and death of photoreceptors and choriocapillaris; in neovascular or wet AMD, loss of choriocapillaris with an intact RPE monolayer in wet AMD has been observed, indicating that the loss of choroidal vasculature may be the initial insult to the RPE/Bruch’s membrane /choriocapillaris complex [74].
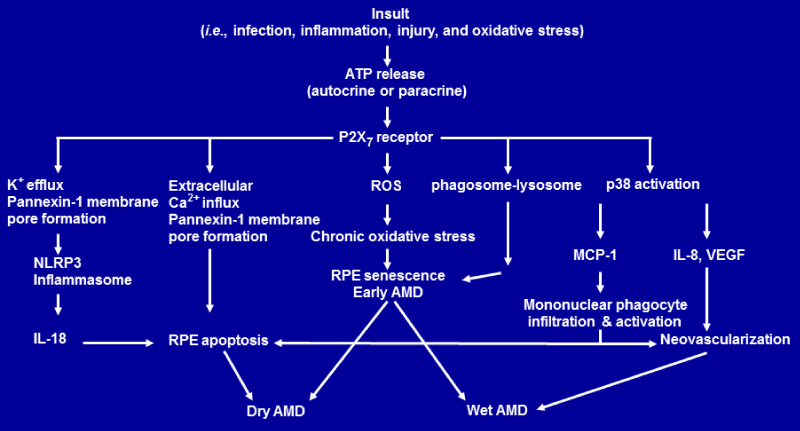
Figure 1b: Proposed model of the P2X7 receptor–mediated signaling that leads to age–related macular degeneration. Schematic diagram shows P2X7 receptormediated signaling pathways that lead to RPE senescence, RPE apoptosis and AMD [17,90,91; 19;37; 36]. Abbreviations Used: AMD, age–related macular degeneration; ATP, adenosine triphosphate; IL–8, interleukin 8; IL–18, interleukin–18; MCP–1, monocyte chemoattractant protein–1; NLRP3, nucleotide–binding domain and leucine–rich repeat containing family, pyrin domain containing 3; RPE, retinal pigmented epithelium; ROS,reactive oxygen species; VEGF, vascular endothelial growth factor.
In neovascular AMD, severe photoreceptor loss develops with sub retinal hemorrhage due to growth and invasion of abnormal and invasion blood vessels. Recently, [36] demonstrate that compared to control vitreous samples, ATP levels in the vitreous samples from AMD patients with sub retinal hemorrhage were increased. In co–culture with primary mouse retinal cells, extra vascular blood induced a massive ATP release and photoreceptor cell apoptosis. Caspase–9 activation and apoptosis–inducing factor translocation from mitochondria to nuclei were also observed [36], indicating involvement of mitochondrial apoptotic pathways in ATP–induced photoreceptor cell apoptosis. BBG, a selective P2X7R antagonist prevents photoreceptor cell apoptosis in a mouse model of sub retinal hemorrhage. These data suggest that activation of P2X7R by extracellular ATP may accelerate photoreceptor cell apoptosis in AMD with sub retinal hemorrhage.
Pyridoxal – phosphate – 6 – azophenyl – 2’, 4’ – disulphonic acid (PPADS) is a non–selective P2 antagonist [75]. A laser induced choroidal neovascularization (CNV) mouse model is usually used as wet AMD model for testing the efficacy of drugs intended to attenuate CNV [76]. After daily topical application of 4.17 mM PPADS for three consecutive days, the area of neovascularization and membrane attack complex deposition were examined in the eye tissues one week later to evaluate for progression of eye tissue damage and blood vessel growth. Birke et al [77] found that topical application of the PPADS attenuated both the area of CNV and membrane attack complex deposition in this mouse model of laser induced CNV. As PPADS inhibited BzATP–induced inward currents in HEK293 cells stably expressing the human recombinant P2X7R [75], we speculate that PPADS could protect retina against membrane attack complex deposition and CNV through inhibiting the activation P2X7R. Further experiments are needed to support this idea.
TheP2X7R is also critical in the development of dry AMD. Using in vitro cultured human RPE model, we found that functional P2X7R is expressed in human RPE cells, and that activation of P2X7R induces human RPE apoptosis that is dependent on P2X7Rmediated extracellular Ca2+ influx [17]. We proposed that abnormal Ca2+homeostasis through the activation of P2X receptors, especially P2X7R could cause the dysfunction and apoptosis of RPE that underlie AMD [17]. Recently, Kerur et al [37] demonstrated a critical role of nuclear factor κB (NF–&kappaB) and P2X7R in mediating Alu RNAinduced RPE degeneration. Alu RNA is a 300 nucleotide, noncoding transcript. It is metabolized by a micro RNA processing enzyme DICER1 into harmless cleavage fragments. DICER1 deficiency results in accumulation of Alu RNAs. This accumulation induces cultured human RPE death and mouse RPE degeneration in vivo [78,79]. BAY 11–7082 (NF–&kappaB inhibitor), A–740003 (P2X7R antagonist; [80]), and glyburide (aninhibitor of nucleotide–binding domain and leucinerich repeat containing family, pyrin domain containing 3 (NLRP3) inflammasome), all protected wild–type mice from Alu RNA–induced RPE degeneration [37]. Alu RNA–induced mouse RPE degeneration was also protected in mice lacking NF–?B gene or P2X7R gene, when compared to wild–type control [37].
Activation of NLRP3 inflammasome in other systems requires at least two signals, a priming signal and an activating signal. A priming signal involves induction of NLRP3 inflammasome components (NLRP3, apoptosis–associated speck–like protein containing a caspase recruitment domain (ASC), and pro–caspase–1)and procytokines (pro–interleukin–1β and pro–interleukin–18).An activating signal promotes the assembly of NLRP3 inflammasome components, proteolytic activation of caspase–1, and processing of pro–cytokines into mature cytokines [32,81,82].The NF–&kappaB family of transcription factors regulates many cellular responses including inflammation and cell death. Both in vitro and in vivo experiments identified that the P2X7R is responsible for ATP–dependent IL–1β release [83– 87]. Disruption of the P2X7R gene abolishes chronic inflammatory [87]. In the RPE, NF–&kappaB is a key transcription factor regulating Alu RNA–induced NLRP3 inflammasome priming; whereas P2X7R is a key protein mediating Alu RNA–induced NLRP3 inflammasome activation and consequent RPE degeneration [37].
The exact mechanisms by which NLRP3 inflammasome is activated remains elusive. The P2X7R activation, generation of reactive oxygen species (ROS), and lysosomal destabilization are among the generally supported mechanisms. P2X7R and ROS are major contributors to the activation of NLRP3 inflammasome in other systems [81,82]. Interestingly, activation of P2X7Ralso leads to ROS production [88,89] in macrophages, and induces lysosomal alkalinization, lipid oxidation, and reduced phagosome clearance in ARPE–19 cells [19]. Therefore, activation of the P2X7R could be the key to activation of NLRP3 inflammasome.
The Proposed Model of the P2X7R–Mediated Signaling that Leads to AMD
Based on recent discoveries discussed above, we propose a model of P2X7R–mediated signaling pathways that lead to RPE senescence, RPE apoptosis and AMD (Figure2). Tissue insult such as infection and inflammation not only can induce ATP release in an autocrine— paracrine manner, but also can promote the expression of P2X7R. If the P2X7Ris activated by the released ATP, it could increase the ROS levels that induce RPE senescence as part of the phenotype of earlyAMD. Activation of P2X7Rcould also lead to lysosome–phagosome dysfunction, RPE apoptosis and geographic atrophy or dry AMD through extracellular Ca2+ influx and pannexin–1 membrane pore formation. Activation of P2X7Rby ATP could also trigger K+ efflux and pannexin–1 membrane pore formation. K+ efflux and pannexin–1 membrane pore formation activate NLRP3 inflammasome. This activation of NLRP3 inflammasome triggers the secretion of IL–18 which induces RPE degeneration and dry AMD.
On the other hand, activation of P2X7Rby ATP can also activate p38 which mediates monocyte chemoattractant protein–1 (MCP–1), interleukin 8 (IL–8) and vascular endothelial growth factor (VEGF) secretion. IL–8 and VEGF promotes vessel growth, leading to wet AMD. MCP–1 can attract and activate mononuclear phagocyte. Activated mononuclear phagocytes can kill RPE cells [90,91], leading to dry AMD. It is also possible that activated mononuclear phagocytes can promote vessel growth, leading to wet AMD [8].
Conclusion
Collectively, recent advances provide greater insight into P2X7R–mediated critical signaling pathways in the RPE and AMD, including(1) the Ca2+–mitochondrial pathway, leading to RPE and photoreceptor apoptosis; (2) the NLRP3 inflammasome pathway, resulting in production and secretion of IL–18; and (3) phagosomelysosome pathway, triggering impaired autophagic degradation.
Several P2X7R antagonists have been demonstrated to be effective for inhibiting or blocking P2X7R–mediated RPE and photoreceptor death and dysfunction in both in vitro and in vivo models of AMD. Moreover, a recent genetic study has demonstrated that a haplotype containing two rare genetic variants of P2X4R and P2X7R is associated with increased susceptibility to AMD [35], underscoring the importance of the P2X7R and the P2X4R in AMD.
In the light of the recent discoveries on the roles of the P2X7R in the RPE and AMD, it is expected that P2X7R– and P2X4R–mediated new and key pathways that contribute to AMD pathogenesis would be identified, providing impetus for the development of preventive and therapeutic strategies for AMD, via targeting P2X7R and P2X4R, their ligands, their downstream pathways and/or protein–protein interactions.
Acknowledgements
This research was supported by a grant from the University of Michigan Claude Pepper Older Americans Independence Center (D. Yang) through NIH P30AG024.
References
- Klein R, Li X, Kuo JZ, Klein BE, Cotch MF. Associations of candidate genes to age-related macular degeneration among racial/ethnic groups in the multi-ethnic study of atherosclerosis. Am J Ophthalmol. 2013; 156: 1010-1020.
- Vanderbeek BL, Zacks DN, Talwar N, Nan B, Musch DC. Racial differences in age-related macular degeneration rates in the United States: a longitudinal analysis of a managed care network. Am J Ophthalmol. 2011; 152: 273-282.
- You QS, Xu L, Yang H, Li YB, Wang S. Five-year incidence of age-related macular degeneration: the Beijing Eye Study. Ophthalmology. 2012; 119: 2519-2525.
- Smith W, Assink J, Klein R, Mitchell P, Klaver CC. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001; 108: 697-704.
- Rudnicka AR, Jarrar Z, Wormald R, Cook DG, Fletcher A. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012; 119: 571-580.
- Priya RR, Chew EY, Swaroop A. Genetic studies of age-related macular degeneration: lessons, challenges, and opportunities for disease management. Ophthalmology. 2012; 119: 2526-2536.
- Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013; 13: 438-451.
- Ardeljan D, Chan CC. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res. 2013; 37: 68-89.
- Cheung LK, Eaton A. Age-related macular degeneration. Pharmacotherapy. 2013; 33: 838-855.
- Evans JR, Lawrenson JG. Dietary interventions for AMD: what do we know and what do we not know? Br J Ophthalmol. 2013; 97: 1089-1090.
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science. 1996; 272: 735-738.
- Sluyter R, Shemon AN, Wiley JS. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1 beta release from human monocytes. J Immunol. 2004; 172: 3399-3405.
- Garcia-Marcos M, Pochet S, Marino A, Dehaye JP. P2X7 and phospholipid signalling: the search of the "missing link" in epithelial cells. Cell Signal. 2006; 18: 2098-2104.
- Barth K, Weinhold K, Guenther A, Young MT, Schnittler H. Caveolin-1 influences P2X7 receptor expression and localization in mouse lung alveolar epithelial cells. FEBS J. 2007; 274: 3021-3033.
- Barth K, Weinhold K, Guenther A, Linge A, Gereke M. Characterization of the molecular interaction between caveolin-1 and the P2X receptors 4 and 7 in E10 mouse lung alveolar epithelial cells. Int J Biochem Cell Biol. 2008; 40: 2230-2239.
- Weinhold K, Krause-Buchholz U, Rödel G, Kasper M, Barth K. Interaction and interrelation of P2X7 and P2X4 receptor complexes in mouse lung epithelial cells. Cell Mol Life Sci. 2010; 67: 2631-2642.
- Yang D, Elner SG, Clark AJ, Hughes BA, Petty HR. Activation of P2X receptors induces apoptosis in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011; 52: 1522-1530.
- Yang D, Cui B, Elner SG, Elner VM. Upregulation of P2X7 receptor expression by aging, lipopolysaccharide and interferon-? in the retinal pigment epithelium. Invest Ophthalmol Vis Sci 2013; 54: 1170.
- Guha S, Baltazar GC, Coffey EE, Tu LA, Lim JC. Lysosomal alkalinization, lipid oxidation, and reduced phagosome clearance triggered by activation of the P2X7 receptor. FASEB J. 2013; 27: 4500-4509.
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004; 240: 31-304.
- Buell GN, Talabot F, Gos A, Lorenz J, Lai E. Gene structure and chromosomal localization of the human P2X7 receptor. Receptors Channels. 1998; 5: 347-354.
- Denlinger LC, Fisette PL, Sommer JA, Watters JJ, Prabhu U. Cutting edge: the nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J Immunol. 2001; 167: 1871-1876.
- Costa-Junior HM, Sarmento Vieira F, Coutinho-Silva R. C terminus of the P2X7 receptor: treasure hunting. Purinergic Signal. 2011; 7: 7-19.
- Wickert LE, Blanchette JB, Waldschmidt NV, Bertics PJ, Denu JM. The C-terminus of human nucleotide receptor P2X7 is critical for receptor oligomerization and N-linked glycosylation. PLoS One. 2013; 8: e63789.
- Di Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995; 16: 524-528.
- Kubo M, Cinader B. Polymorphism of age-related changes in interleukin (IL) production: differential changes of T helper subpopulations, synthesizing IL 2, IL 3 and IL 4. Eur J Immunol. 1990; 20: 1289-1296.
- Miller RA. Aging and immune function. Int Rev Cytol. 1991; 124: 187-215.
- Hobbs MV, Weigle WO, Noonan DJ, Torbett BE, McEvilly R . Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993; 150: 3602-3614.
- Riancho JA, Zarrabeitia MT, Amado JA, Olmos JM, González-Macías J. Age-related differences in cytokine secretion. Gerontology. 1994; 40: 8-12.
- Chen J, Mo R, Lescure PA, Misek DE, Hanash S. Aging is associated with increased T-cell chemokine expression in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2003; 58: 975-983.
- Lin T, Walker GB, Kurji K, Fang E, Law G. Parainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: implications for age-related degenerative diseases of the eye. Cytokine. 2013; 62: 369-381.
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006; 176: 3877-3883.
- Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006; 442: 527-532.
- Takenouchi T, Sekiyama K, Sekigawa A, Fujita M, Waragai M. P2X7 receptor signaling pathway as a therapeutic target for neurodegenerative diseases. Arch Immunol Ther Exp (Warsz). 2010; 58: 91-96.
- Gu BJ, Baird PN, Vessey KA, Skarratt KK, Fletcher EL. A rare functional haplotype of the P2RX4 and P2RX7 genes leads to loss of innate phagocytosis and confers increased risk of age-related macular degeneration. FASEB J. 2013; 27: 1479-1487.
- Notomi S, Hisatomi T, Murakami Y, Terasaki H, Sonoda S. Dynamic increase in extracellular ATP accelerates photoreceptor cell apoptosis via ligation of P2RX7 in subretinal hemorrhage. PLoS One. 2013; 8: e53338.
- Kerur N, Hirano Y, Tarallo V, Fowler BJ, Bastos-Carvalho A. TLR-independent and P2X7-dependent signaling mediate Alu RNA-induced NLRP3 inflammasome activation in geographic atrophy. Invest Ophthalmol Vis Sci. 2013; 54: 7395-7401.
- Soto F, Garcia-Guzman M, Karschin C, Stühmer W. Cloning and tissue distribution of a novel P2X receptor from rat brain. Biochem Biophys Res Commun. 1996; 223: 456-460.
- Murrell-Lagnado RD, Qureshi OS. Assembly and trafficking of P2X purinergic receptors (Review). Mol Membr Biol. 2008; 25: 321-331.
- Ma W, Korngreen A, Weil S, Cohen EB, Priel A. Pore properties and pharmacological features of the P2X receptor channel in airway ciliated cells. J Physiol. 2006; 571: 503-517.
- Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol. 2007; 72: 1447-1456.
- Casas-Pruneda G, Reyes JP, Pérez-Flores G, Pérez-Cornejo P, Arreola J. Functional interactions between P2X4 and P2X7 receptors from mouse salivary epithelia. J Physiol. 2009; 587: 2887-2901.
- Craigie E, Birch RE, Unwin RJ, Wildman SS. The relationship between P2X4 and P2X7: a physiologically important interaction? Front Physiol. 2013; 4: 216.
- Denlinger LC, Coursin DB, Schell K, Angelini G, Green DN. Human P2X7 pore function predicts allele linkage disequilibrium. Clin Chem. 2006; 52: 995-1004.
- Stokes L, Fuller SJ, Sluyter R, Skarratt KK, Gu BJ. Two haplotypes of the P2X(7) receptor containing the Ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1beta secretion. FASEB J. 2010; 24: 2916-2927.
- Sakaki H, Fujiwaki T, Tsukimoto M, Kawano A, Harada H. P2X4 receptor regulates P2X7 receptor-dependent IL-1β and IL-18 release in mouse bone marrow-derived dendritic cells. Biochem Biophys Res Commun. 2013; 432: 406-411.
- Wiley JS, Gu BJ. A new role for the P2X7 receptor: a scavenger receptor for bacteria and apoptotic cells in the absence of serum and extracellular ATP. Purinergic Signal. 2012; 8: 579-586.
- Ferguson TA, Green DR. Autophagy and phagocytosis converge for better vision. Autophagy. 2014; 10: 165-167.
- Dutot M, Liang H, Pauloin T, Brignole-Baudouin F, Baudouin C. Effects of toxic cellular stresses and divalent cations on the human P2X7 cell death receptor. Mol Vis. 2008; 14: 889-897.
- Petrovski G, Berényi E, Moe MC, Vajas A, Fésüs L. Clearance of dying ARPE-19 cells by professional and nonprofessional phagocytes in vitro- implications for age-related macular degeneration (AMD). Acta Ophthalmol. 2011; 89: e30-34.
- Humphreys BD, Dubyak GR. Induction of the P2z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-gamma in the human THP-1 monocytic cell line. J Immunol. 1996; 157: 5627-5637.
- Humphreys BD, Dubyak GR. Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol. 1998; 64: 265-273.
- Gudipaty L, Humphreys BD, Buell G, Dubyak GR. Regulation of P2X(7) nucleotide receptor function in human monocytes by extracellular ions and receptor density. Am J Physiol Cell Physiol. 2001; 280: C943-953.
- Jiang LH. Inhibition of P2X(7) receptors by divalent cations: old action and new insight. Eur Biophys J. 2009; 38: 339-346.
- Stojilkovic SS, Leiva-Salcedo E, Rokic MB, Coddou C. Regulation of ATP-Gated P2X Channels: From Redox Signaling to Interactions with Other Proteins. Antioxid Redox Signal. 2013; .
- Nihei OK, Savino W, Alves LA. Procedures to characterize and study P2Z/P2X7 purinoceptor: flow cytometry as a promising practical, reliable tool. Mem Inst Oswaldo Cruz. 2000; 95: 415-428.
- Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011; 63: 641-683.
- Khakh BS, North RA. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron. 2012; 76: 51-69.
- Miller CM, Boulter NR, Fuller SJ, Zakrzewski AM, Lees MP. The role of the P2X₇ receptor in infectious diseases. PLoS Pathog. 2011; 7: e1002212.
- Sim JA, Young MT, Sung HY, North RA, Surprenant A. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci. 2004; 24: 6307-6314.
- Adriouch S, Dubberke G, Diessenbacher P, Rassendren F, Seman M. Probing the expression and function of the P2X7 purinoceptor with antibodies raised by genetic immunization. Cell Immunol. 2005; 236: 72-77.
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002; 82: 1013-1067.
- Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA. Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem. 2006; 49: 3659-3666.
- Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br J Pharmacol. 2009; 157: 1203-1214.
- Rakoczy PE, Lai CM, Baines M, Di Grandi S, Fitton JH. Modulation of cathepsin D activity in retinal pigment epithelial cells. Biochem J. 1997; 324 : 935-940.
- Rakoczy PE, Sarks SH, Daw N, Constable IJ. Distribution of cathepsin D in human eyes with or without age-related maculopathy. Exp Eye Res. 1999; 69: 367-374.
- Chen CS, Chen WN, Zhou M, Arttamangkul S, Haugland RP. Probing the cathepsin D using a BODIPY FL-pepstatin A: applications in fluorescence polarization and microscopy. J Biochem Biophys Methods. 2000; 42: 137-151.
- Kim JY, Zhao H, Martinez J, Doggett TA, Kolesnikov AV . Noncanonical autophagy promotes the visual cycle. Cell. 2013; 154: 365-376.
- Das S, Seth RK, Kumar A, Kadiiska MB, Michelotti G, et al.Purinergic receptor X7 is a key modulator of metabolic oxidative stress-mediated autophagy and inflammation in experimental nonalcoholic steatohepatitis.Am J PhysiolGastrointest Liver Physiol. 2013; 305: 950-963.
- Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004; 122: 598-614.
- Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006; 38: 450-471.
- Gehrs KM, Jackson JR, Brown EN, Allikmets R, Hageman GS. Complement, age-related macular degeneration and a vision of the future. Arch Ophthalmol. 2010; 128: 349-358.
- Detrick B, Hooks JJ. Immune regulation in the retina. Immunol Res. 2010; 47: 153-161.
- Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med. 2012; 33: 295-317.
- Chessell IP, Michel AD, Humphrey PP. Effects of antagonists at the human recombinant P2X7 receptor. Br J Pharmacol. 1998; 124: 1314-1320.
- Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res. 2010; 29: 500-519.
- Birke K, Lipo E, Birke MT, Kumar-Singh R. Topical application of PPADS inhibits complement activation and choroidal neovascularization in a model of age-related macular degeneration. PLoS One. 2013; 8: e76766.
- Kaneko H, Dridi S, Tarallo V, Gelfand BD, Fowler BJ. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011; 471: 325-330.
- Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012; 149: 847-859.
- Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J PharmacolExpTher. 2006; 319: 1376-1385.
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010; 140: 821-832.
- Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012; 13: 333-342.
- Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J Immunol. 1996; 156: 1531-1539.
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997; 159: 1451-1458.
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001; 276: 125-132.
- Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002; 168: 6436-6445.
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005; 114: 386-396.
- Noguchi T, Ishii K, Fukutomi H, Naguro I, Matsuzawa A. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J Biol Chem. 2008; 283: 7657-7665.
- Moore SF, MacKenzie AB. NADPH oxidase NOX2 mediates rapid cellular oxidation following ATP stimulation of endotoxin-primed macrophages. J Immunol. 2009; 183: 3302-3308.
- Yang D, Elner SG, Lin LR, Reddy VN, Petty HR. Association of superoxide anions with retinal pigment epithelial cell apoptosis induced by mononuclear phagocytes. Invest Ophthalmol Vis Sci. 2009; 50: 4998-5005.
- Yang D, Elner SG, Chen X, Field MG, Petty HR. MCP-1-activated monocytes induce apoptosis in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011; 52: 6026-6034.
- Skarratt KK, Fuller SJ, Sluyter R, Dao-Ung LP, Gu BJ. A 5' intronic splice site polymorphism leads to a null allele of the P2X7 gene in 1-2% of the Caucasian population. FEBS Lett. 2005; 579: 2675-2678.
- Cabrini G, Falzoni S, Forchap SL, Pellegatti P, Balboni A. A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J Immunol. 2005; 175: 82-89.
- Roger S, Mei ZZ, Baldwin JM, Dong L, Bradley H. Single nucleotide polymorphisms that were identified in affective mood disorders affect ATP-activated P2X7 receptor functions. J Psychiatr Res. 2010; 44: 347-355.
- Gu BJ, Sluyter R, Skarratt KK, Shemon AN, Dao-Ung LP. An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J Biol Chem. 2004; 279: 31287-31295.
- Sun C, Chu J, Singh S, Salter RD. Identification and characterization of a novel variant of the human P2X(7) receptor resulting in gain of function. Purinergic Signal. 2010; 6: 31-45.
- Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem. 2006; 281: 2079-2086.
- Gu BJ, Zhang W, Worthington RA, Sluyter R, Dao-Ung P. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem. 2001; 276: 11135-11142.
- Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem. 2003; 278: 17108-17113.
- Husted LB, Harsløf T, Stenkjær L, Carstens M, Jørgensen NR. Functional polymorphisms in the P2X7 receptor gene are associated with osteoporosis. Osteoporos Int. 2013; 24: 949-959.
- Jørgensen NR, Husted LB, Skarratt KK, Stokes L, Tofteng CL. Single-nucleotide polymorphisms in the P2X7 receptor gene are associated with post-menopausal bone loss and vertebral fractures. Eur J Hum Genet. 2012; 20: 675-681.
- Wesselius A, Bours MJ, Henriksen Z, Syberg S, Petersen S, et al. Association of P2X7 receptor polymorphisms with bone mineral density and osteoporosis risk in a cohort of Dutch fracture patients. Osteoporos Int. 2013; 24: 1235-1246.
- Gidlöf O, Smith JG, Melander O, Lövkvist H, Hedblad B. A common missense variant in the ATP receptor P2X7 is associated with reduced risk of cardiovascular events. PLoS One. 2012; 7: e37491.
- Areeshi MY, Mandal RK, Panda AK, Haque S. Association of P2X7 A1513C (rs3751143) gene polymorphism with risk of tuberculosis: evidence from a meta-analysis. Genet Test Mol Biomarkers. 2013; 17: 662-668.