Case Report
Austin J Clin Ophthalmol. 2014;1(3): 1015.
Laser Photocoagulation Sparing the Papillomacular Bundle for Peripapillary Polypoidal Choroidal Vasculopathy Lesions
Fumio Shiraga1*, Yukari Shirakata2, Chieko Shiragami2, Ayana Yamashita2, Atsushi Fujiwara1, Mio Hosokawa1, Shuhei Kimura1 and Yuki Morizane1
11Department of Ophthalmology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Japan
22Department of Ophthalmology, Kagawa University Faculty of Medicine, Japan
*Corresponding author: Fumio Shiraga, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, 2-5-1 Shikata-cho Kitaku Okayama 700-8558, Japan
Received: February 14, 2014; Accepted: March 12, 2014; Published: March 21, 2014
Abstract
Purpose: To evaluate the safety and effectiveness of laser photocoagulation for indocyanine green angiography (ICGA)–identified peripapillarypolypoidal choroidal vasculopathy (PPCV) lesions.
Design: Retrospective, interventional, consecutive case series.
Methods: Twenty–two consecutive PPCV eyes with serous retinal detachments in the macula, which were treated with direct laser photocoagulation,were retrospectively reviewed. No patient had undergone previous treatment for PCV. All 22 eyes were treated with multicolor red (659 nm) laser photocoagulation. The laser spot was focused on the retinal pigment epithelium underneath the detached retina to spare the papillomacular bundle. Laser photocoagulation targeted the entire ICGA–identified lesion, including both the polypoidal lesions and the abnormal network.
Results: The mean follow–up period after the first treatment was 27.2 months (range, 6–77 months). The logMAR BCVA was stable or improved by ≥0.3 logMAR in 19 eyes (86.4%). Three cases had recurrent leakage requiring retreatment, resulting in visual acuity loss ≥0.3 logMAR. Eighteen (81.9%) of the 22 studied eyes required no additional treatment during follow–up.
Conclusion: For PPCV, laser photocoagulation was effective in maintaining or improving visual acuity with only a single treatment session, since laser photocoagulation was administered for all vascular lesions, sparing the papillomacular bundle.
Keywords: Polypoidal choroidal vasculopathy; Peripapillary lesion; Laser photocoagulation; Papillomacular bundle; Perimetry.
Introduction
Polypoidal choroidal vasculopathy (PCV) is a distinct clinical entity associated with multiple recurrent serosanguineous detachments of the retinal pigment epithelium and neurosensory retina, caused by leakage and bleeding from a peculiar choroidal vascular lesion [1]. Indocyanine green angiography (ICGA) can clearly demonstrate these characteristic changes of PCV in the choroidal vasculature as branching vascular networks with polypoidal dilatations at the terminals of the network beneath the retinal pigment epithelium [2]. The incidence of PCV in Japanese appears to be high (54.7% of neovascular AMD); the polypoidal lesions appear to have a predilection for the macular area and are relatively rarely detected in the peripapillary area in Japanese patients [3,4].
Peripapillary PCV (PPCV) frequently causes serous⁄ hemorrhagic neurosensory retinal detachments in the macular area, and the prospect for visual recovery is uncertain and often poor. Although photodynamic therapy (PDT) with verteporfin has been used to stabilize the vision [5–8], PDT is not applicable to PPCV because the lesions are close to the optic disc. Intravitreal vascular endothelial growth factor (VEGF) antagonist monotherapy may require constant treatment regimens and frequent intravitreal injections, because it seems to be ineffective for diminishing choroidal vascular changes [9,10]. However, frequent injections may increase the risks of endophthalmitis and systemic side effects.
Laser photocoagulation is suitable for the treatment of PCV lesions located in the extrafoveal area. As demonstrated by Yuzawa et al in 2003, which evaluated the efficacy of photocoagulation for 47 PCV eyes [11], several reports of laser photocoagulation for PCV have been described, and these results have been variable [11–13]. Direct laser photocoagulation may cause unfavorable side effects, such as retinal pigment epithelium rupture and subretinal or vitreous hemorrhage. In addition, direct photocoagulation for PPCV may damage the papillomacular bundle.
The purpose of this study was to evaluate the safety and effectiveness of laser photocoagulation of ICGA–identified PPCV lesions.
Patients and Methods
Twenty–two treatment–naïve eyes of 21 consecutive Japanese patients (14 men, 7 women) with PPCV and serous neurosensory retinal detachment (SRD) in the macular area, treated with direct laser photocoagulation between July 2003 and December 2011, were retrospectively reviewed. They were followed–up for at least 6 months after photocoagulation. No patient had undergone previous treatment for PCV. The potential risks and benefits were explained in detail, and written, informed consent was obtained from all patients. The exclusion criteria included: previous treatment for PCV, such as laser photocoagulation, submacular surgery, and PDT; glaucoma; tears in the retinal pigment epithelium (RPE); and the presence of macular diseases causing visual loss, such as diabetic maculopathy, retinal vascular occlusion, or idiopathic juxtafoveal macular telangiectasia. The treatment was approved by the Institutional Review Board⁄Ethics Committee at Kagawa University Faculty of Medicine.
The best–corrected visual acuity (BCVA) measured with a standard decimal VA chart was recorded, and the mean BCVA was calculated using the logarithm of the minimum angle of resolution (logMAR) scale. All patients underwent a standard examination including slitlamp biomicroscopy using a 78–D lens, fundus color photography, imaging and fluorescein angiography (FA) using the Topcon TRC– 50DX fundus camera (Topcon, Tokyo, Japan), ICGA, and spectral domain optical coherence tomography (SD–OCT) using a confocal scanning laser ophthalmoscope (Spectralis™⁄HRA Heidelberg Retina Angiograph 2, Heidelberg Engineering, Heidelberg, Germany). The visual fields were assessed by a Humphrey Field Analyzer (HFA, Humphrey Instruments, Germany) in seven eyes and/or MAIA (MAIA; CenterVue, Topcon Japan, Tokyo, Japan) in six eyes. MAIA is a nonmydriatic, near–infrared, line–scanning, laser ophthalmoscope, which incorporates a high–frequency eye tracker and an automated threshold fundus microperimeter.
The diagnosis of PCV was made based on ICGA findings. FA and ICGA were performed to identify polypoidal lesions and abnormal vascular networks, as well as to determine the location and activity of the PCV vascular lesions. FA and ICGA were performed before treatment and when there was recurrence of exudative and/or hemorrhagic manifestation. SD–OCT was performed before and at every visit after treatment to evaluate the morphologic changes of the retina. Regular follow–up examinations were performed at 1 month, 3 months, 6 months, and 1 year after photocoagulation and every 6 months thereafter, and also when patients noticed symptoms. In 7 of the 22 eyes, HFA and/or MAIA was performed at the last visit to measure retinal sensitivity influenced by laser photocoagulation.
All 22 eyes were treated with laser photocoagulation using a multicolor red (659 nm) laser photocoagulation system (Novus Varia, Lumenis Japan, Tokyo, Japan). Patients were treated using the slitlamp microscope with a Goldmann’s three mirror contact lens (with a spot–size magnification factor of 1× to focus the laser. The following laser settings were used: pulse duration, 0.2 s; spot size, 200 µm; power, 200 to 350 mW (until a ‘grayish–white’ lesion was attained). The laser spot was focused on the retinal pigment epithelium underneath the detached retina. Photocoagulation targeting the whole lesion, including both the polypoidal lesions and the abnormal network, was performed because the vascular lesion was present outside the fovea.
Baseline and post–treatment values were compared using the paired t–test, with P values < 0.05 considered statistically significant. All statistical analyses were performed using SPSS for Windows, Version 17.0 (SPSS, Inc., Chicago, IL). Data are presented as means ± SD.
Results
Patient characteristics
Twenty–two eyes of 21 patients who had completed at least 6 months of follow–up were included in this study. There were 14 men 66.7%) and 7 women (33.3%). The mean age at baseline was 72.9 years, with a range of 56 to 83 years. The patients underwent their first laser photocoagulation for PPCV at our hospital. The follow–up period after the first photocoagulation ranged from 6 to 77 months, with a mean of 27.2 months. At baseline, all 22 eyes showed SRD including the fovea, resulting from leakage from the lesion. The characteristics and clinical data of the 21 patients (22 eyes) at baseline and after treatment are shown in the Table.
Visual outcomes
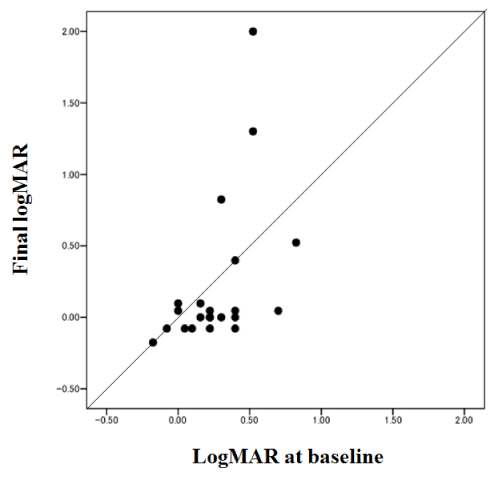
The logMAR BCVA (mean ± standard deviation) was 0.27 ± 0.25 at baseline compared with 0.22 ± 0.53 at the last visit; the difference was not significant (paired t–test). The logMAR BCVA was stable or improved by ≥ 0.3 logMAR in 19 eyes (86.4%). In three eyes, logMAR BCVA deteriorated by ≥ 0.3 logMAR; these three cases had recurrences of leakage and needed retreatment during the follow–up period, resulting in visual acuity loss ≥ 0.3 logMAR. In all 12 eyes with visual acuity better than 20⁄40 at baseline, the visual acuity was maintained or improved. The final logMAR VA was significantly correlated with the logMAR at baseline (Pearson’s correlation test, p=0.021, r=0.488) (Figure1).
Anatomic changes
At baseline, all 22 eyes showed SRD in the macula including the fovea, resulting from leakage from the lesion. The vascular lesion was present outside the foveal vascular zone in all 22 eyes. SRD disappeared within one month after photocoagulation. In 18 eyes (81.8%), recurrence of subretinal fluid, leakage, bleeding, or subsequent development of classic CNV did not occur during the follow–up period after a single laser treatment.
Figure 1 :Changes in visual acuity (the logarithm of the minimum angle of resolution (logMAR). The mean VA was 0.54 at baseline and 0.60 at the last visit. The visual acuity was maintained or improved after treatment in 19 eyes (86.4%).
In all 22 eyes, SD–OCT, done at the last visit, showed that the laser–induced damage was limited to retinal pigment epithelium and photoreceptors only within the treatment area, sparing the retinal nerve fiber layer.
Functional changes
In the seven eyes examined with HFA, HFA showed that laserinduced deterioration of retinal sensitivity was limited within the treatment area, and damage to the papillomacular bundle was not detected. In the six eyes examined with MAIA, the decrease in retinal sensitivity was consistent with the treatment area, and deterioration of the visual field due to a damaged papillomacular bundle was not detected.
Retreatment
When there was a recurrence of leakage on OCT, FA and ICGA were performed. Four eyes (18.2%) underwent additional therapy during the follow–up period. In one eye (case 15) with a recurrence of leakage without distinct classic choroidal neovascularization (CNV), IVR was applied three times and the exudative manifestation resolved. In three patients, classic CNVs involving the fovea developed. These three eyes were treated with standard–fluence PDT alone, because these recurrences developed before the introduction of IVR.
Case Report
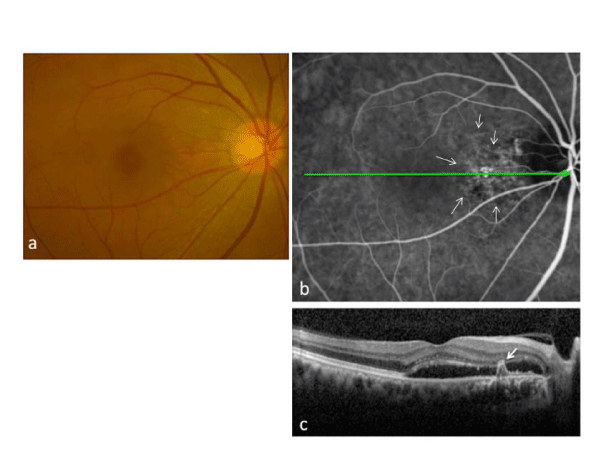
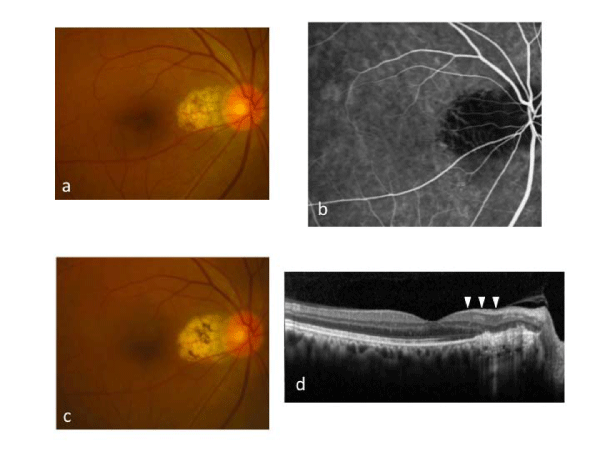
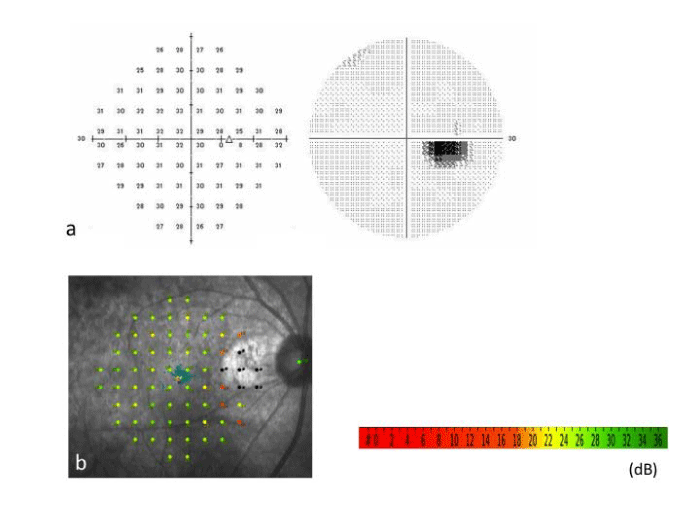
Case 13: A 65–year–old man with SRD in the macular area of the right eye was treated with laser photocoagulation of PPCV vascular lesions (Figures 2–4). At baseline (Figure 2), the best–corrected visual acuity (BCVA) was 20⁄25 in the right eye. A polypoidal lesion and abnormal vascular network were seen in the peripapillary area (Figure 2a). Early–phase ICGA before treatment showed an abnormal vascular network and polypoidal lesions in the peripapillary area (Figure 2b). OCT demonstrated SRD and a protrusion of retinal pigment epithelium resulting from the polypoidal lesions (arrow) (Figure 2c). Multicolor red laser photocoagulation was applied for all ICGA–identified vascular lesions composed of polypoidal lesions and the abnormal vascular network. The following laser settings were used: pulse duration, 0.2 s; spot size, 200 Ám; power, 200 to 250 mW. At one month after treatment, SRD disappeared, and the retinal pigment epithelium protrusion became flat. There was a laser scar at the peripapillary area (Figure 3a). On ICGA, the polypoidal lesions and vascular network were totally occluded (Figure 3b). The BCVA was improved to 20/16 and maintained for 17 months until the final visit. There was no recurrence of polypoidal lesions and no apparent atrophic creep of the laser scar for 17 months (Figure 3c); no additional treatment was administered over 17 months. SD–OCT at 17 months did not demonstrate any subretinal fluid or pigment epithelial detachment, and the nerve fiber layer of the papillomacular bundle was not thinned (Figure 3d). Although there was a visual field defect consistent with only the irradiated area, retinal sensitivity in the macula was maintained in the HFA (Figure 4a). MAIA also showed that the retinal sensitivity in the macula was good, and fixation was excellent (Figure 4b).
Figure 2: Case 13: A 65-year-old man was treated with laser photocoagulation of peripapillary polypoidal choroidal vasculopathy lesions. At baseline, polypoidal lesions (arrows in Figure 2b) are seen in the peripapillary area, and serous retinal detachment (SRD) is seen in the macula (Figure 2a). Early phase indocyanine green angiography before treatment shows an abnormal vascular network and polypoidal lesions in the peripapillary area (Figure 2b). Spectral-domain optical coherence tomography demonstrates SRD and a protrusion of retinal pigment epithelium resulting from polypoidal lesions (arrow)(Figure 2c).
Figure 3:Case 13: After a single treatment with multicolor red laser photocoagulation for peripapillary polypoidal vasculopathy. The laser scar is seen in the peripapillary area at 3 months (Figure3a). Early-phase indocyanine green angiography shows the complete occlusion of both the polypoidal lesions and the vascular network at 3 months (Figure 3b). There was no recurrence of polypoidal lesion and no enlargement of the laser scar at 17 months (Figure 3c). On spectral domain optical coherence tomography at 17 months, neither subretinal fluid nor pigment epithelial detachment is seen, and the nerve fiber layer (arrowheads) is not thinned (Figure 3d).
Figure 4: Case13: There is a visual field defect (blind spot enlargement) consistent with the irradiated area, but the retinal sensitivity in the macula is intact in the Humphrey Field Analyzer at 17 months (Figure 4a). Microperimetry (MAIA) also shows that the retinal sensitivity in the macula is good, and the fixation is excellent (Figure 4b).
Discussion
Many different treatment modalities have been used in an attempt to preserve vision for PCV. Currently, the main modalities for PCV are PDT [5,14,15] or anti–VEGF therapy [9,10,16] alone or in combination [17,18].
The incidence of PCV in Japanese is high (54.7% of neovascular AMD), and polypoidal lesions very frequently appear in the macular area(93% ) [3,4,19]. Although PPCV is relatively rarely detected in Japanese patients(7%) [3,4], their SRDs often extend to the macular area. In patients with PPCV complicated by SRD affecting the fovea, the visual prognosis is often poor. Although PCV generally responds well to PDT, PDT is not applicable for PPCV, because the vascular lesion of PPCV is located close to the optic disc. VEGF concentrations in the aqueous were found to be markedly higher in eyes with PCV than in normal controls [20]. However, intravitreal VEGF antagonists might reduce the fluid from PCV, but they were ineffective for diminishing the choroidal vascular changes [9,10]. In addition, intravitreal VEGF antagonist monotherapy may require constant treatment regimens and frequent intravitreal injections. Frequent injections may increase the risks of endophthalmitis and systemic side effects. Furthermore, the combination of frequent visits and high cost is a burden to patients.
Laser photocoagulation was used in the treatment of polypoidal lesions. Yuzawa et al. [11] evaluated the efficacy of photocoagulation for PCV. They concluded that laser photocoagulation is recommended if it can treat the entire polypoidal lesion, because laser photocoagulation leaving a vascular network leads to the recurrence of SRD originating from remaining or newly formed polypoidal lesions and/or secondary CNV. In the same way, when PDT is applied to PCV, the remaining vascular network is associated with recurrence of PCV [21]. Since abnormal vascular networks usually persist after PDT, their enlargement and neovascular changes result in a high frequency of recurrent polypoidal lesions on long–term follow–up [22,23]. If the abnormal vascular networks of PCV remain after treatment, recurrence might be inevitable. Therefore, since the occlusion of not only polypoidal lesions but also abnormal vascular networks is ideal to prevent recurrence, laser photocoagulation should be applied to the entire PPCV lesion. In the present study, after complete photocoagulation, recurrence of CNV occurredin only 4 eyes (18.2%). No additional treatment was needed in the follow–up period in 18 eyes (81.8%).
By detailed examination of the findings on SD–OCT, an intact retinal nerve fiber layer, including the papillomacular bundle, was observed in the irradiated area after laser photocoagulation. In the current study, laser photocoagulation targeting the entire lesion under SRD was performed with a long wavelength laser (689 nm). Both targeting the lesion underneath the subretinal fluid and irradiating the lesion with a long wavelength laser may prevent damage to the inner retina, including the retinal nerve fiber layer. This is essential to laser photocoagulation for PPCV lesions located beneath the papillomacular bundle. In the present study, although only seven eyes were examined with HFA and⁄or MAIA, the intact retinal nerve fiber layer confirmed on SD–OCT corresponded to the outcome on HFA and/or MAIA. Neither HFA nor MAIA showed a visual field defect due to a damaged retinal nerve fiber layer.
In 19 eyes (86.4%), visual acuity was maintained or improved in the current study. The remaining eyes with development of classic CNV underwent additional standard–fluence PDT because IVR was not permitted in Japan at that time. If IVR had been permitted, the visual acuity might have been maintained. In addition, the reason why the mean visual acuity was not significantly improved might be good visual acuity at baseline in most of the studied eyes. Indeed, 14 (63.6%) of the studied 22 eyes had a good visual acuity of 20⁄40 or better at baseline, and 6 eyes had a visual acuity of 20⁄25 or better. However, all 12 eyes with a visual acuity better than 20⁄40 at baseline maintained a good visual acuity.
In conclusion, the current study showed that laser photocoagulation for PPCV using a multicolor red laser was effective in maintaining or improving visual acuity with only a single treatment session, since laser photocoagulation was administered to all vascular lesions with sparing of the papillomacular bundle. Laser photocoagulation of the entire vascular lesion could be a beneficial treatment for PPCV even in the current anti–VEGF era. Single lasertreatment may be less invasive than monthly anti–VEGF treatments.
References
- Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990; 10:1–8.
- Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995; 15: 100-110.
- Uyama M, Matsubara T, Fukushima I, Matsunaga H, Iwashita K. Idiopathic polypoidal choroidal vasculopathy in Japanese patients. Arch Ophthalmol. 1999; 117: 1035-1042.
- Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007; 144: 15-22.
- Chan WM, Lam DS, Lai TY, Liu DT, Li KK. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology. 2004; 111: 1576-1584.
- Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2008; 115: 141-146.
- Akaza E, Yuzawa M, Matsumoto Y, Kashiwakura S, Fujita K. Role of photodynamic therapy in polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2007; 51: 270-277.
- Sayanagi K, Gomi F, Sawa M, Ohji M, Tano Y. Long-term follow-up of polypoidal choroidal vasculopathy after photodynamic therapy with verteporfin. Graefes Arch Clin Exp Ophthalmol. 2007; 245: 1569-1571.
- Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol. 2010; 94: 297-301.
- Hikichi T, Ohtsuka H, Higuchi M, Matsushita T, Ariga H. Improvement of angiographic findings of polypoidal choroidal vasculopathy after intravitreal injection of ranibizumab monthly for 3 months. Am J Ophthalmol. 2010; 150: 674-682.
- Yuzawa M, Mori R, Haruyama M. A study of laser photocoagulation for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2003; 47: 379-384.
- Nishijima K, Takahashi M, Akita J, Katsuta H, Tanemura M. Laser photocoagulation of indocyanine green angiographically identified feeder vessels to idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol. 2004; 137: 770-773.
- Lee MW, Yeo I, Wong D, Ang CL. Argon laser photocoagulation for the treatment of polypoidal choroidal vasculopathy. Eye (Lond). 2009; 23: 145-148.
- Ogino T, Takeda M, Imaizumi H, Okushiba U. Photodynamic therapy for age-related macular degeneration in Japanese patients: results after one year. Jpn J Ophthalmol. 2007; 51: 210-215.
- Yamashita A, Shiraga F, Shiragami C, Ono A, Tenkumo K. One-year results of reduced-fluence photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2010; 149: 465-471.
- Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Oshima Y. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2008; 92: 70-73.
- Sato T, Kishi S, Matsumoto H, Mukai R. Combined photodynamic therapy with verteporfin and intravitreal bevacizumab for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2010; 149: 947-954.
- Ruamviboonsuk P, Tadarati M, Vanichvaranont S, Hanutsaha P, Pokawattana N. Photodynamic therapy combined with ranibizumab for polypoidal choroidal vasculopathy: results of a 1-year preliminary study. Br J Ophthalmol. 2010; 94: 1045-1051.
- Sho K, Takahashi K, Yamada H, Wada M, Nagai Y. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003; 121: 1392-1396.
- Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol 2006; 141:456–462.
- Oishi A, Mandai M, Kimakura M, Nishida A, Kurimoto Y. Characteristics of fine vascular network pattern associated with recurrence of polypoidal choroidal vasculopathy. Eye (Lond). 2011; 25: 1020-1026.
- Wakabayashi T, Gomi F, Sawa M, Tsujikawa M, Tano Y. Marked vascular changes of polypoidal choroidal vasculopathy after photodynamic therapy. Br J Ophthalmol. 2008; 92: 936-940.
- Akaza E, Yuzawa M, Mori R. Three-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2011; 55: 39-44.