Research Article
Austin J Clin Ophthalmol. 2014;1(4): 1017.
Corneal Injuries and Wound Healing – Review of Processes and Therapies
Benjamin D Ashby, Qian Garrett and Mark DP Willcox*
Department of Optometry and Vision Science, University of New South Wales, Australia
*Corresponding author: Mark Willcox, School of Optometry and Vision Science, University of New South Wales, Sydney, NSW 2052, Australia
Received: February 14, 2014; Accepted: March 12, 2014; Published: March 21, 2014
Abstract
Wounds in the cornea are usually self–limiting, but are common and a major reason for visits to hospital emergency departments. Although most heal without permanent visual impairment and do not require hospitalisation, in Australia these injuries are conservatively estimated to cost $155 million per year when lost working days and medical expenses are both taken into consideration. There is also some limited evidence that these injuries have a longer term impact on quality of life. This paper describes the impact of corneal injuries, the structure of the cornea, the cellular processes involved in corneal wound healing, and review the current and future therapies to treat this condition.
Introduction and Background
Epidemiology
Eye injuries are the most common reason for attendance at an emergency department, accounting for 14% of eye department presentations and 8% of eye department hospitalisations [1,2]. These figures are reflected in an annual incidence of 0.315% (CI 0.266– 0.363%) based on over 800,000 patients treated in the United States for eye injuries in emergency departments in the year 2000 [3]. This is of a similar magnitude to the findings of most other large studies of urban populations in developed countries over the last 40 years although reported values are approximately three times higher for Australian and Italian populations [4–7]. These numbers increase in rural locations [6] and developing [8] or newly industrialised countries[7]. Approximately three quarters of these emergency ocular trauma assessments are for corneal foreign bodies or an abrasion indicating the primary site of injuries is most commonly the corneal epithelium.[2,3,6].
Less common but with far greater likelihood of sight threatening sequelae are chemical burns which account for 5–22% of eye injuries [3,6,9,10]. Based on hospital admissions the majority, 30–80%, of burns are from alkaline substances. These come into contact with the eye from either accidental exposure or assaults [9,11]. The most common sources of alkali chemicals involved in ocular burns are readily available building products and household cleaners such as ammonia, sodium hydroxide, plaster and cement [9,12,13].
Rarer still and also of a severe nature is corneal compromise associated with microbial keratitis (MK), that is a particular concern in contact lens wearing populations. The incidence in Australia has recently been reported at 4.2 per 10,000 (CI 3.4–5.5) [14] a figure comparable to the 4–5 per 10,000 found in most international studies in developed countries over the last 30 years [15–17]. This problem is however not limited to contact lens wears as the remaining 78% of hospital treated MK have no association with lens use, although this only represents an incidence of 5–11 per 100,000 in the general population [13,18]. The problem is much greater in developing countries where the incidence is higher by a factor of 10 [19].
There is also corneal compromise occurring as part of elective procedures, primarily the incisions and ablations created for refractive and cataract surgeries. Since gaining regulatory approval in the US in 1998 over a million patients a year are undergoing laser assisted in–situ keratomileusis (LASIK) procedures, with smaller numbers undergoing the more invasive photorefractive keratectomy (PRK) and the increasingly rarer radial keratotomy [20, 21]. While cataract extractions require a smaller epithelial wound they are performed much more frequently than refractive surgeries with an increasing majority of incisions positioned over the clear cornea rather than the limbus [21]. According to World Health Organisation data most developed countries have a cataract surgery rate of over 4,000 per year per 100,000 population with Australia approximately 50% higher based on data reported in the Blue Mountains Eye Study [22].
Morbidity
Although ocular traumas account for almost 2% of emergency hospital presentations, most heal without permanent visual impairment and do not require hospitalisation [1]. Those of working age are consistently reported to be at more than double the risk for these eye injuries with an average of three lost days of productivity [2]. This is a considerable economic burden that is only increased by the additional care required for the 5–11% of cases that will need hospitalisation [2,3,6]. In Australia these injuries are conservatively estimated to cost $155 million per year when lost working days and medical expenses are both taken into consideration [6].
There is also some limited evidence that these injuries have a longer term impact on quality of life. A study of 47 patients conducted 2 years after an ocular trauma found twice the level of physiological morbidity expected in the normal population. A fear of blindness was reported by 87% of patients and according to the authors the experience caused “considerable and sustained distress many months after the trauma”. This is disproportionate to the outcome as only 17% of participants had a final vision of less than 6⁄60 in the injured eye [23]. The psychological impact may be explained by the considerable pain, photophobia and sudden reduction in vision associated with corneal injuries. A study of 88 consecutive patients presenting to casualty with corneal abrasions rated their pain, photophobia and visual blur as 3.7, 3.5 and 2.8 out of 5, respectively, on a visual analogue scale where 0 is no pain⁄photophobia⁄blur and 5 is the worst pain⁄ photophobia⁄blur imaginable [24]. At the time of presentation half of these patients required both oral analgesia in addition to topical ocular pain control measures such as lubricants and cycloplegia. The pain is then reported to diminish as epithelialisation takes place [24– 27]. Thus initially corneal injury is associated with appreciable pain and reduced vision that then improved as healing takes place. Even after the eye has recovered there may still be a prolonged period of anxiety.
Chemical injuries represent a much more serious threat to the eye with 8–12% requiring hospital admission for a week followed by 3–6 months of treatment [6]. For non–admitted cases the majority still need medical reviews for 1–7 days [9]. Of the corneal traumas the prognosis is poorest for alkali burns with 20% resulting in a final visual acuity of less than 6⁄12 [11,28].
Corneal compromise from microbial invasion is a rare but serious event with an incidence in the world developed countries of 2 million every year [29]. Mild microbial keratitis associated with contact lens use has been estimated to cost AU$1228 (US$1353) per case. This includes both the cost of treatment and lost working days [30]. The economic impact of microbial keratitis in non–contact lens wearers from developed economies has not been reported. In developing countries, using India as an example, microbial keratitis results in an average of one month lost productivity, a cost of treatment that exceeds the average monthly income and a final visual outcome of less than 6⁄60 in almost half of cases [31]. A lower cost may be anticipated for non–contact lens wearers as they would not incur the $150 expense for new spectacles [32]. Also the incidence of the more aggressive Pseudomonas aeruginosa pathogen is much lower [13]. owever a higher cost in the non–contact lens wearing population is more likely as the average disease severity is greater, with longer hospital stays and more medical re–examinations required [13]. More than half of those cases of a severity requiring hospital admission result in a permanent reduction in vision [33].
Cataract surgery in the current era is a safe procedure with severe, sight threatening, adverse events occurring in no more than 0.4% of cases [34]. Of these complications endophthalmitis accounts for 1⁄3rd with a proposed link to the corneal incision that is supported by double the rate of infection for the more popular clear corneal incisions versus scleral incisions [34–36]. For refractive surgery LASIK is currently preferred over PRK due to the faster visual recovery and lower level of postoperative pain [21,37,38]. Both of these disadvantages to PRK can be attributed to the increased healing time required by a largerarea of cornea to reepithelialise after the procedure. PRK is however suitable for a wider range of patients and is reported to provide a better visual outcome with fewer complications [39]. This is however not universally agreed upon with a pre–2005 meta–analysis of the literature by Shortt et al. tentatively suggesting LASIK may be safer but of similar efficacy [40].
Corneal Wound Healing
Corneal Structure: The cornea is the transparent anterior segment of the globe of the eye that refracts light onto the retina. It is the first element in the eye’s optical system, contributing 2⁄3rd of the eye’s total focusing power and must remain essentially clear for optimal vision to be achieved. To maintain transparency the cornea must remain a vascular, unscarred and preserve its highly regular organised internal structure. The cornea is also the most sensitive tissue in the body.
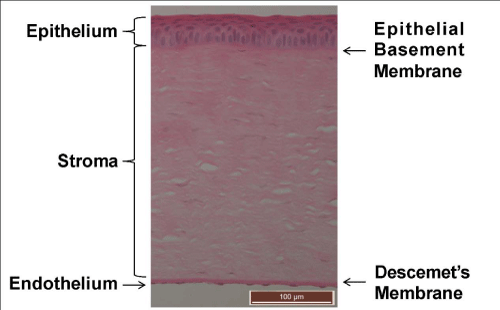
A normal cornea consists of three well defined layers (Figure 1).
Figure 1 :Light micrograph (20x objective) of the transverse section of a Guinea pig cornea stained with haematoxylin and eosin. Nuclei can be visualised in blue while cytoplasm and collagen appear pink. Epithelial cell nuclei appear blue surrounded by pink cytoplasm within the epithelial layer. Keratocyte nuclei are seen as long thin blue structures within the pink lamella of stromal collagen. Endothelial cell nuclei are seen in a single layer beneaththe deeply pink stained Descemet’s membrane.
The cellular epithelium is the most external layer of the cornea, underlying this is the collagenous stroma that is bounded internally by the cellular endothelium (Figure 1) [41]. These layers extend laterally to the limbus which marks the transition from corneal epithelium to conjunctival epithelium anteriorly, corneal stroma to sclera centrally and corneal endothelium to trabecular meshwork on the posterior surface [42]. Externally the epithelium is coated by a thin, continuously renewed layer of tears while internally the endothelium is in constant contact with the aqueous humour that fills the anterior chamber of the eye [41].
Tear Film: The tear film is a thin fluid layer of approximately 10 µm coating the surface of the cornea [43]. Tears are formed by secretions from the lacrimal glands with contributions from the meibomian glands and goblet cells [43]. These are mixed and spread over the cornea by the regular action of the blink. The tear film serves the epithelium by preserving its optical quality, delivering nutrients, facilitating the transport of signalling proteins, providing a pathway for access of leukocytes and acting as a reservoir for topically applied medications [44]. Additionally the richness of the tears in antimicrobial factors and the high frequency of tear film renewal help to protect the cornea from infection and harmful substances [45].
Corneal Epithelium: The major roles of the corneal epithelium are to act as a barrier, assist in maintaining a constant level of stromal hydration and to serve as an optical interface. The barrier function of the epithelium is directed against physical trauma, pathogens and chemicals. All of these are a threat to the underlying stroma that may lose its transparency if damaged. By preventing the diffusion of water and solutes from the tear film into the cornea the epithelium also enables stromal deturgescence (relative dehydration of the stroma) to persist. In remaining smooth and clear the epithelium transmits refracted light from the tear film uninterrupted into the eye [46].
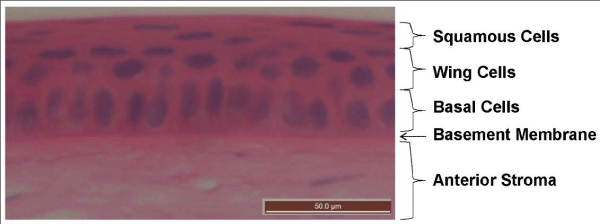
The corneal epithelium consists of five to seven layers of non–keratinised squamous stratified type epithelial cells with an approximate thickness of 50 µm. The cells of this layer can be distinguished morphologically into basal columnar cells, wing cells and superficial squamous cells. Basal cells form a monolayer that rests atop the basal laminar. They are the deepest and tallest cells in the epithelium accounting for 40% of the total thickness (Figure 2).
Figure 2: Light micrograph (60x objective) of the transverse section of a Guinea pig corneal epithelium and anterior stroma stained with haematoxylin and eosin. Nuclei can be visualised in blue while cytoplasm and collagen appear pink. The tall basal cell nuclei are seen in a single layer above the dark pink stained basement membrane. Nuclei of wing cells can be visualised as two layers of round to horizontal oval structures. The flattened nuclei of squamous cells can be observed in the three most superficial layers.
These give rise to the adjacent anterior two to three layers of wing cells that are less tall than the basal cells but not as elongated as the squamous cells of the layer above. The wing cells will in turn become squamous cells, forming the external–most two to three layers of flattened cells. The squamous cells of the ocular surface desquamate relatively frequently to be washed away into the tear film over the course of the blink. The transition of a cell from basal, to wing, then squamous cell that is shed takes one to two weeks. This regular turn over contributes to the protective role of the epithelium as any infected or damaged cells are quickly replaced (Figure 2) [41,47,48].
Epithelial cells attach to each other and the extracellular matrix though junctional and non–junctional adhesions. Junctional adhesions are structures where cytoskeletal, transmembrane, and in some cases extracellular proteins, aggregate to form a complex with distinct morphology under the electron microscope. In the corneal epithelium there are zonula occludens junctions, gap junctions, adherens junctions, desmosomes and hemidesmosomes. Nonjunctional adhesions are ligand–receptor interactions that are not visible without immunochemical staining.
The surface exposed apical aspect of the squamous cells express glycocalyx that interacts with tear film mucin to maintain the cornea’s wettability. This supports an even distribution of tear film across the surface of the cornea. Cells of this layer are terminally differentiated and will desquamate into the tear film within a week. Squamous corneal epithelial cells of the superficial layers form zonula occludens junctions with surrounding cells contributing significantly to the epithelium’s barrier function [49]. This junction forms a complete anastomosis of adjoining cell membranes and closes the paracellular pathway, preventing the movement of fluid and hydrophilic substances into the cornea [50].
Wing cells express the protein precursors required for the formation of tight junctions when they transition to superficial squamous cells [51]. These cells are joined to each through abundant desmosomes that connect plaques attached to the cytoskeleton via transmembrane bridging proteins [52]. Adherens junctions are also present between wing cells, as they are for all layers of the epithelium. Like desmosomes these junctions connect the cytoskeleton of adjacent cells but connect to actin rather than keratin filaments [53]. The intercellular junctions of the wing cells contribute significantly to structural integrity of the corneal epithelium.
The basal cells are the only mitotic layer in the corneal epithelium with their stem cells believed to be located in the limbal region [54]. As a basal cell divides it gives rise to a daughter cell that moves upwards and towards the central cornea as it differentiates into a wing cell [55]. Basal cells are interconnected by gap junctions that maintain a fixed distance between adjacent cells and form pores through which the cytoplasm of adjacent cells can communicate [51,56]. These junctions are believed to be important to cell differentiation and development [57]. Hemidesmosomes are cell to substrate adhesion junctions that anchor the basal cells to the stroma and basal lamina [58]. They are located within the basal aspect of the cell and are associated with cytoskeletal and transmembrane proteins complexes. These complexes attach anchoring filaments, that join to anchoring fibrils in the basement membrane which in turn terminate in anchoring plaques located 0.6 µm beyond the epithelial basement membrane within the stroma, firmly fixing the epithelium to the underlying cornea [59].
In addition to the junctional adhesions of the hemidesmosomes there are also non–junctional attachments. Integrin heterodimers are the major receptor for both these types of cell–substrate with a large extra–cellular domain and smaller transmembrane and cytoplasmic domains. Ligands for the integrins are present on collagen, laminin, vitronectin and fibronectin [60].
Epithelial basement membrane is a 0.2 µm thick layer separating the cellular epithelium from the stroma (Figure 2) [61]. It is primarily composed of collagen type IV, the sulphated proteoglycan heparin, laminin and ectactin [62]. Kalinin, a laminin isoform, within the basement membrane forms strong attachments to the cell membrane protein integrin α6β4 subunit of hemidesmosomes and to collagen VII of anchoring fibrils [63,64]. This secures the basal layer of cells to the basal lamina and the underlying stroma.
Stroma: The stoma accounts for approximately 80% of the corneal thickness (Figure 1) increasing slightly towards the limbus. It is composed of approximately 200 parallel laminae of densely packed connective tissue with relatively few cells [41]. The laminae of predominantly type I–type V collagen heterodimer fibrils are of a consistent diameter and regularly spaced by their association with sulphated proteoglycans [65,66]. Most studies report type III collagen in these fibrils [67, 68] although some investigators only detected type III at the limbus and in the sclera [69]. These proteoglycans are relatively hygroscopic, thus making the stroma prone to swelling, however to maintain its transparency the stroma must remain at a constant thickness and in a deturgescent state [70]. This is achieved by the tight junctions of the epithelium anteriorly and the leaky tight junctions combined with the ion pump of the endothelium posteriorly. This structure prevents a net influx of fluid from either surface [70]. The tensile strength required to maintain the shape of the cornea against the intraocular pressure and resist trauma arises from the collagen networks of the stroma [71]. In the human stroma the anterior 18 µm of these collagen fibres are irregularly packed into an acellular layer referred to as Bowman’s layer [72].
A fine syncytium of cells known as keratocytes are interspersed sparsely between the collagen sheets of the stoma maintaining and remodelling surrounding collagen [73]. Keratocytes are a quiescent type of fibroblast that form interconnecting networks joined through long dendritic processes [74]. By light microscopy of transverse corneal sections keratocytes have a long thin profile (Figure 1) compressed between laminae. It is only in coronal sections that it becomes apparent that the keratocytes are 10–20 times greater in width than height [74]. Keratocytes express matrix metalloproteases (MMP), glycosaminoglycans (GAG), collagen and crystallins important to their role in stromal homeostasis, transparency and healing [41,75].
Endothelium and Descemet’s Membrane: Descemet’s is the collagenous basement membrane of the endothelium. It is secreted by the endothelial cells onto the posterior stroma, reaching a thickness of up to 10 µm [76]. The endothelial cells form a monolayer upon Descemet’s membrane appearing flat in transverse sections (Figure 1) but hexagonal when viewed from above, forming a honeycomblike network. In humans this layer becomes non–proliferative after birth. Endothelial cells are joined by gap junctions and “leaky” tight junctions [77]. These “leaky” junctions allow the movement offluid between the stroma and anterior chamber, a process vital for nutrient supply to the stroma. The ion pumps of the endothelial cells continuously transport ions from the stroma into the aqueous humour; this precedes the osmotic movement of fluid from the stroma into the hypertonic aqueous humour. This mechanism acts o move fluid in the opposite direction to the imbibition pressure of the stromal proteoglycans and the balance of these two mechanisms is vital for the maintenance of a clear, relatively dehydrated, cornea [78].
Wound Healing
Epithelium: As the external layer of the cornea the exposure of the epithelium makes it the most likely cell population to be damaged. Fortunately it is also the layer that heals most quickly and most effectively returns to it pre–injury structure and function. Occasionally the epithelium will be compromised by a wound that is slow to heal. This can occur in wounds caused by alkali burns [79], infections [80] or wounds associated with diabetic neuropathy [81]. The greatest risk under these circumstances is to the more vulnerable, deeper structures of the cornea. Fortunately the majority of epithelial wounds heal rapidly and without consequence.
Corneal epithelial wound healing can be divided into four phases with specific physiological functions [82]. The first phase is referred to as the latent phase as there is no cell movement or change in cell numbers [83]. During this time there is an increase in metabolic activity and a reorganising of the cell structure in preparation for the next phase [84]. The second phase is migration, this is characterised by the cells surrounding the wound sliding over and covering the denuded surface [85]. This is followed by the proliferation phase where the cells begin to divide and differentiate, restoring the epithelium’s original structure and intercellular junctions [51,86]. The final phase is the return of the cell–substrate attachments present in non–motile epithelium [87]. Often a subsequent phase will begin before the completion of the prior phase however the sequence of the phases is maintained. Thus in the corneal epithelium wound healing progresses through four overlapping phases that first enable cell movement then coverage of the wound area before restoring the original cell density and reforming cell attachments.
Phase One: Latency: The lag phase is a delay immediately following corneal epithelial injury while the cells and the ocular surface transform to facilitate cell migration. Those cells that were damaged by the wound stimulus undergo apoptosis and are soon shed into the tear film [83,88]. Fibronectin polymerises onto the wound bed forming a provisional extracellular matrix over which the cells can later more easily move [89]. Adherens junctions and gap junctions are lost and desmogleins of the desmosomes are remodelled [51]. Basal cells in the area surrounding the wound dismantle their hemidesmosome attachments to the substrate [90]. This involves the redistribution of α6β4 integrin, normally localised within the hemidesmosome complexes, across the basal aspect of the cell membrane where it forms a weaker non–junctional cell–substrate attachment to the laminin of the basement membrane [91,92]. At this time integrin α5β1 and αvβ6 receptors are up–regulated and expressed on the basal aspect of the cell membrane [93,94]. Intracellularly, cytoskeletal vinculin and the contractile actin stress fibre terminals localise to the α5β1 receptors to form junctional complexes called focal contacts concentrated along the wound margin [95–97]. This phase may continue without the initiation of migration for up to several hours [83]. Thus during the lag phase several of the intercellular junctions are dismantled, the cell–substrate junctions are replaced with weaker attachments and a provisional extracellular matrix is laid down in preparation for cell migration.
Phase Two: Migration: During the migration phase cells move over the wound area to cover the defect. Following the delay of the latent phase cells at the wound margin flatten and spread into a monolayer [83]. Those cells at the leading edge of the wound are observed to send out filopodia and have the characteristic ruffled appearance of migrating epithelia [83,98]. Focal contacts bind to ligands on the fibronectin provisional matrix and cell movement is enabled by the cytoskeletal contractile mechanisms of the actin stress fibres interacting with the adhesion complexes [95, 96, 99,100]. By electron microscopy it appears that neighbouring cells remain attached by desmosomes as the epithelium sides as a sheet across the wound bed to cover the denuded area, pulled by the cells at the leading edge of the wound where focal contacts are most concentrated [85,95]. Following this single layer of sliding cells a multilayered mass movement of cells has also been observed [46,85]. In an uncomplicated epithelial lesion the cells migrate across the wound bed at the constant rate of 0.05–0.06 mm⁄h with some slowing as closure is approached [83,101,102]. To sustain this migration protein synthesis and in particular glycoprotein synthesis continues from the latency stage and peaks in this phase [84,103]. While these cells are migrating there is no mitotic activity in or around the wound area [86]. Thus in the cell migration phase the leading edge pulls a layer of epithelium across the provisional matrix to rapidly cover the wound in a process that is independent of cell proliferation.
Phase Three Proliferation: Cell proliferation restores the epithelial cell density and occurs in conjunction with cell differentiation. Mitosis is observed to be delayed within the wound area until after the cell migration phase is complete [86]. Cell mitosis is thus initially limited to the basal transient amplifying cells that are distant from the wound and to the limbus where the stem cells are believed to be located [86,104]. During wound healing more than half of the normally slow cycling stem cells of the limbal region are induced to proliferate while the transient amplifying cells of the basal layer proliferate more frequently with an increased number of divisions before terminal differentiation [105]. This causes a 5 fold increase in proliferation in the limbal region and 3 fold increase in the peripheral cornea that peaks at approximately 24 hours after wounding [104,106]. As the area of proliferation progresses centrally, daughter cells are displaced inwards and upwards towards the more superficial layers, differentiating into wing then squamous cells to restratify the epithelium [86,107]. The first junctions to reform are the zonula occludens, appearing behind the leading edge of the wound, restoring the epithelial barrier function even before migration is complete [51,108]. The basement membrane is remodelled by the migrated epithelium, secreting laminin within 24 hours that can attach to integrin αvβ6 of the cell membrane [51,94]. This coincides with the reassembly of gap junctions, adherens junctions and desmosomes directly above [51]. Thus the proliferation phase commences with cells outside the wound area increasing their mitotic activity and the daughter cells beginning to differentiate as they repopulate the epithelium while within the wound area intercellular junctions are re–established as a new basement membrane is secreted.
Phase Four: Attachment: The attachment phase re–establishes a firm adherence of the epithelial layer to the underlying substrate. Hemidesmosomes are the basis of the strong attachment of the basal epithelial cells to the basement membrane and stroma [91,109]. For wounds where the basement membrane remains intact hemidesmosomes reform over pre–existing anchoring fibrils [109]. When the basement membrane and stroma have been damaged or removed the anchoring fibril and the hemidesmosome are resynthesised simultaneously progressing from below the wound margin towards the central cornea [110]. This process begins once the overlying epithelium has stratified but may not be complete until up to a year after the original injury [87]. In the absence of the hemidesmosome the overlying epithelium is prone to sloughing and recurrent erosions [87]. Thus solid re–attachment of the regenerated epithelium to the underling stroma via hemidesmosomes is the final stage in epithelial wound healing and may evolve over a prolonged period if the anterior stroma is compromised.
Stromal wound healing: Stromal wound healing involves transformation of keratocytes, production of fibrous material and tissue remodelling [111]. The stromal injury response begins with epithelial compromise leading to swelling of the stroma, apoptosis of fibroblasts and invasion of inflammatory cells and this may occur even without penetration to the stroma [112,113]. Early in the process leucocytes are recruited from the conjunctival vessel to the site of injury where they serve to protect against infection but can also contribute to tissue damage [113,114]. There is also evidence that suggests they are involved in the promotion of healing [115]. The keratocytes nearestthe wound apoptose and those adjacent to this area are activated to become fibroblasts or myofibroblasts [116,117]. These transformed cells then migrate to the wound and deposit fibrotic material to fill the defect [118,119]. This material may contract and opacify which reduces corneal transparency and can alter the refractive curvature of the cornea. The number of transformed keratocytes start to decline once the wound is filled and a slow remodelling phase takes place over weeks to years in an attempt to reorganise the collagen to restore transparency [117]. Depending on the balance of mediators, MMPs and the extent of the wound, the outcome of stromal healing can be regeneration of normal stromal structure, an opaque scar, stromal melting or neovascularisation [120–123]. Stromal wound healing involves transformed keratinocytes repairing the wound with fibrousmaterial in the presence of inflammatory cells and may result in reduced vision.
Endothelium and Descemet’s Membrane: Endothelial wound healing is limited to reorganisation of the remaining cells and secretion of a new basement membrane. The endothelium may be damaged by either penetrating injuries, trans–corneal incisions or excessive corneal distortion [41]. Damage to the endothelial disrupts the process that moves fluid out of the stroma resulting in swelling and a loss of transparency in the area of the wound [124]. Unlike the Guinea pig and rabbit, the cells of the human corneal endothelium do not undergo mitosis in vivo. When endothelial cell loss occurs in the human eye the wound is filled by the migration of adjacent cells and an enlarging of the remaining cells to occupy a greater surface area [125– 127]. This differs from the rabbit where endothelial wound healing is predominantly by mitosis and the Guinea pig where this is in addition to a fibro–cellular response in severe injury [128]. Endothelial cell migration begins within six hours and progresses at approximately 1 mm⁄day [129,130]. Within a week of the endothelium reforming over the posterior stroma, normal function is restored causing any swelling to subside [129]. If Descemet’s membrane is breached this is repaired by the endothelium secreting a new basement membrane [131]. Endothelial wound healing is entirely by cell migration in the human eye with normal function returning a number of days after coverage of the area by cells.
Analysis and interpretation
Endogenous Modulation of Wound Healing: Corneal wound healing is a highly complex and tightly co–ordinated physiological response that is modulated by a number of signalling pathways and processes. Cytokines released during injury play a key role in the orchestration of the wound healing response, particularly interleukin (IL) 1 and IL–6. These cytokines are able to influence the expression of the growth factors such as epithelial growth factor (EGF), keratocyte growth factor (KGF), hepatocyte growth factor (HGF), transforming growth factor (TGF) and platelet derived growth factor (PDGF) [132]. These growth factors and cytokines together regulate the healing processes of apoptosis, migration, proliferation and differentiation. Epithelial intracellular signalling pathways that have been implicated in wound healing process include activation of the mitogen-activated protein kinases (MAPK), phosphatidylinositol 3–kinase (PI-3K), Rho family and protein kinase C (PKC) [51,107,133]. Closure of the wound also involves activation of proteases, changes to extracellular matrix proteins and is influenced by neural factors [51]. Thus wound healing in the corneal epithelium is regulated by a range of cytokines, growth factors, matrix proteins and proteases.
Cytokines are released following corneal epithelial injury and appear to initiate the healing response. IL–1, IL–6 and TNF-α are expressed when the epithelium is injured [134]. The levels of IL–1 and IL–6 are found to be proportional to the severity of the injury [134]. IL–6 facilitates epithelial cell migration by upregulation of the integrin receptor for fibronectin and may also influence production of fibrotic material by activated keratocytes [118,135]. IL–1 has been shown to promote wound healing synergistically with EGF in addition to upregulating the levels of HGF and KGF [136,137]. IL–1 also stimulates other stromal changes such as MMP production, keratocyte apoptosis and leukocyte infiltration [138–140]. The angiogenic and neutrophil chemotactic factor IL–8 is also up-regulated by IL–1[141,142]. To a lesser extent than IL–1, injury to the epithelium increases expression of TNF–α that similarly up–regulates IL–8, promotes apoptosis, recruits leukocytes and may influence epithelial healing [134,143– 146]. Thus cytokines released following epithelial injury promote epithelial migration, up-regulate growth factors and mediate stromal healing responses.
Growth factors released in response to epithelial injury or following cytokine stimulation increase the rates of cell mitosis and migration. HGF, KGF and their epithelial receptors are upregulated in response to IL–1, which increases the rates of epithelial cell proliferation [147,148]. In vitro findings that stimulated HGF production is greatest in corneal keratocytes located centrally, compared to KGF that is greatest in limbal keratocytes, suggest their effects are greatest on basal transient amplifying cells and stem cells, respectively, however in situ hybridisation of healing corneas has not supported this hypothesis [149,150]. Nerve growth factor (NGF) is also up-regulated following injury and has been shown to promote epithelial proliferation and differentiation [151,152]. EGF is an established mitogen of corneal epithelia expressed only at low levels in corneal epithelium with lacrimal gland levels up–regulated during the healing process [148,153,154]. As receptor expression remains unchanged after injury, although increased activation is observed, it has been suggested the role of EGF relates more to epithelial homeostasis rather than repair [148,155]. By comparison other members of the EGF family, heparin–binding EGF–like growth factor (HB–EGF) and TGF-α, are up–regulated in wound healing but have been shown in vitro to only promote epithelial cell migration and inhibit differentiation [147,156]. Similarly promoting epithelial migration is PDGF released from the epithelium and this additionally stimulates stromal keratocytes to migrate and proliferate [157–159]. TGF–β production is also localised to the epithelium but enters the stroma when the basement membrane is damaged, influencing keratocyte phenotype change [160,161]. TGF–β also antagonises the mitogenic action of EGF, KGF and HGF in corneal epithelium suggesting that its activation at the leading edge of the wound may be responsible for locally inhibiting proliferation in migrating epithelial cells [162]. Thus growth factors that are up–regulated by injury promote epithelial migration and modulate proliferation in addition to influencing the stromal response [150,158].
Binding of growth factors and cytokines to cell membrane receptors activates intracellular signalling pathways that direct the cell’s response during healing. Following injury stimulation of epithelial EGF receptors initiates the MAPK and PI–3K intracellular signalling pathways while blocking this receptor or these pathways has an inhibitory effect on cell migration and proliferation [163–168]. In response to attachment of integrins to extracellular matrix proteins or growth factors, including PDGF, members of the Rho family of guanosine triphosphatases (GTPase) promote cell migration through their regulation of cytoskeletal actin polymerisation required for the formation of lamellipodia filopodia, stress fibres and focal adhesions [167,169–174]. Later in the healing process Rho GTPase is involved in the assembly of adherens junctions and gap junctions and cell proliferation [167,173,175]. Binding of HGF and KGF to their receptors stimulates intracellular signalling through MAPK, PI–3K and PKC [176–178]. Isoenzymes of PKC selectively promote either migration or proliferation and while HGF can stimulate both of these, KGF only activates the migration signal [178]. This may contribute to regional differences in migration and proliferation activation during corneal epithelial healing. Thus the binding of growth factors and extracellular matrix ligands activates intracellular signals viaRho GTPase, MAPK, PI–3K or PKC to promote cell migration and proliferation.
The nervous system also contributes to the process of corneal epithelial wound healing. The importance of neural inputs to the corneal wound healing process is suggested by the delayed wound healing that is characteristic of altered trigeminal nerve function associated with herpetic eye disease, fifth nerve lesions, topical anaesthetic abuse and diabetes mellitus [179]. The co–culture of corneal epithelial cells with trigeminal or sympathetic neurons results in increased mitotic activity [180]. Cultured trigeminal neurons also cause epithelial cells to differentiate and express type VII collagen found in anchoring fibrils [180–182]. Addition of the neuropeptide substance P, in conjunction with insulin–like growth factor (IGF), promotes epithelial migration with increased phosphorylation of signalling proteins that are associated with focal adhesion complexes [183,184]. On the other hand, the neuropeptide calcitonin gene related peptide reduces epithelial mitosis in vitro [185]. Noradrenaline from sympathetic nerves may have a minor role in wound healing however to date reports are conflicting [186,187]. Acetylcholine may also have a role in promotion of wound healing, although it is produced in higher levels by the corneal epithelium than parasympathetic nerves at the ocular surface [188,189]. Thus the slow healing occurring in ocular neural pathology and the promotion of wound closure by neural derived factors suggests a role for the nervous system in wound closure.
Extracellular matrix interactions with the epithelium may regulate phases of corneal healing. The finding that focal contact proteins are up–regulated in the presence of fibronectin suggests that provisional extracellular matrix not only facilities cell migration but contributes to its initiation [46]. As a precursor of the mitogen antagonist, TGF–β, is concentrated in the subepithelial extracellular matrix from where its activation and liberation by migrating cell during healing could account for inhibition of proliferation in these cells [161]. Also the appearance of the integrin ligand laminin under migrating corneal epithelium coincides with the re–establishment of intercellular junctions, suggesting interactions between the basement membrane and epithelial cells may regulate the formation of intercellular junctions, as has been demonstrated in other tissues [51, 190, 191]. Thus there is circumstantial evidence that interactions between the epithelium and extracellular matrix proteins regulate aspects of wound healing.
Wound healing is dependent on the activity of proteases. Migration of corneal epithelial cells requires their attachment to the extracellular matrix be temporarily broken. Expression of the serine protease urokinase-type plasminogen activator (uPA) has been noted at the leading edge of epithelial wounds in vitro [192]. In ex vivo eyes the addition of antibodies against uPA or protease inhibitors resulted in dose dependent inhibition of cell migration [192,193]. uPA is able to activate plasminogen, the zymogen of plasmin, present in the stroma, tear film and at the leading edge of wounds [192,194,195]. The serine protease plasmin in turn degrades the fibronectin [196,197]. provisional matrix and laminin [198] of the basement membrane [46,51]. This likely disrupts the cells attachment to the substrate facilitating migration. Plasmin has also been shown to activate TGF–β and MMP–1 that respectively influence mitosis and extracellular matrix remodelling [199,200]. MMP–9 is localised to the leading edge of the wound where it breaks down collagen and basement membrane proteins, modulates IL–1, and activates TGF–β [201–204]. The presence of MMP-9 has been show to regulate the rate of wound closure by reducing mitotic activity, delaying inflammation and then removing the provisional matrix following wound closure [204,205]. Members of the A-disintegrin-and-MMP (ADAM) family have been shown to promote epithelial healing by releasing HB-EGF from corneal epithelial cell membranes following injury [163]. Thus proteases promote and regulate cell migration and proliferation during corneal epithelial wound healing.
Therapeutics for Promotion of Corneal Wound Healing
Given the frequency of corneal epithelial injury [1], the associated pain [24] and the risk of vision loss when healing is delayed [206] there is considerable need for therapeutic agents that are able to support the healing process. To date a wide range of substances have been investigated. The classes of these agents can be generally divided into growth factors, cytokines, proteases, cationic peptides, antioxidants, proteins, anti–inflammatories and surgeries (Table 1). In order to better understand these potential therapies, and their clinical application, it is useful to look at how they promote wound healing. The basic mode of action by which re-epithelialisation is accelerated by these agents is either an increase in the cell’s proliferation rate (PR) and⁄or migration rate (MR). They may also aid wound healing by creating a more favourable environment through secondary mechanisms such as modulating inflammation, countering toxic substances, or facilitating attachment of cells at the completion of wound closure. The level of evidence supporting these agents should also be considered. In order of increasing quality for clinical usefulness the extent of investigation varies from in vitro testing with cultured cells, to in vivo animal model, then once the drug is tested clinically, to individual case reports, case series, randomised controlled trial (RCT) and finally a meta–analysis of multiple RCTs. Also to be factored in are the potential limitations of the therapy beyond its promotion of wound healing. This may include unacceptable side effects, difficulty in making the treatment available, or that investigation of the substance is still at a preliminary stage. Ultimately it is desirable to determine which agent has the greatest promotion of healing for a particular condition. However for agents that have not been compared head–to–head in studies it is very difficult to make judgements of this nature. This is particularly difficult when studies use different species which may have disparate responses to identical therapies [207]. Table 1 summarises the major studies in support of the various agents that have been investigated for their corneal epithelial wound healing properties for different wound types [11, 123, 135, 136, 138, 141, 142, 148, 152, 154, 159, 172, 177, 183, 188, 204, 208–364]. While the relative promotion of healing over the control is reported for studies with a p<0.05, unless otherwise stated, it is acknowledged that comparisons between studies may not be meaningful as in addition to the factors included in the table there is also considerable influence from differences in drug dosage regimes [365], data collection time–points [79] and method of quantifying the healing response [101].
Name of the ongoing trials on aflibercept
ClinicalTrials.gov identifiers
Clinical condition
ATLAS
NCT01773954
Age related macular degeneration (AMD)
ROLL
NCT01670162
Persistent Pigment epithelial Detachments in AMD
EVEN
NCT01722656
Submacular Vascularized Pigment Epithelial Detachment
NVAMD*
NCT01712035
AMD
NEWTON
NCT01870427
Previously Treated Macular Edema Associated With Central Retinal Vein Occlusions
ARChiMEDES
NCT01857544
Recalcitrant Central Retinal Vein Occlusion Associated Macular Edema Despite Prior Anti-VEGF Therapy.
VIVID EAST
NCT01783886
Diabetic Macular Edema (DME) With Central Involvement
VIVID DME
NCT01331681
DME
VISTA DME
NCT01363440
DME
Protocol T†
NCT01627249
DME
ACT
NCT01813773
Proliferative Diabetic Retinopathy
HANDLE
NCT01790893
Presumed ocular histoplasimosis
NCT01724554
Capillary Non-Perfusion
EPIC
NCT01871376
Polypoidal ChoroidalVasculopathy With Hemorrhage or Exudation
Table 1: Potential therapeutics for promotion of corneal epithelial wound healing.
Growth Factors
EGF, as with other growth factors that have been studied for their therapeutic effect in promoting corneal epithelial wound healing, is produced locally in ocular tissues and has a homeostatic role in the maintenance of the ocular surface [155]. Studies on the wound healing properties of these growth factors have demonstrated that several are able to promote either proliferation or migration of corneal epithelial cells (Table 1). Unfortunately a number of these growth factors also have other growth–like effects that may be unwanted in a therapeutic agent used on the cornea. For example, while TGF–β2 accelerated wound closure [214] it also has angiogenic properties [215]. Other wound healing growth factors such as FGF [223] over stimulate the healing process to the point of promoting stromal fibrosis [225]. PDGF–BB promotes cell migration [237] and proliferation [238] but requires the presence of fibronectin [159]. The majority of growth factors have only been tested in animal models (Table 1) although NGF and autologous serum has been the subject of case series’ using lesions unresponsive to conventional therapy. These appear promising but without a trial using a control it is difficult to interpret the clinical utility [232]. EGF however has been tested in a multicentre randomised controlled trial (RCT) [208] and while it was found to accelerate wound closure in patients with abrasions there are questions on the quality of the newly formed epithelial attachments when it is used for alkali burns [209].
Cytokines and Signalling Proteins
Cytokines are signalling molecules that trigger changes in cells of the body. The designation as either a cytokine or growth factor is often historical and based on what the predominant function of the protein appeared to be around the time of its naming. It is now recognised that a number of members from each group have both signalling and growth regulating functions. As with the growth factors most of the cytokines that have been investigated for wound healing are naturally occurring in the eye. IL–1, for example, has been proposed as the aster regulator of the corneal response to injury by modulating keratocyte apoptosis, leukocytes infiltration and angiogenesis [385]. In vitro studies of IL–1 have shown it promotes corneal epithelial cell migration independently of its capacity to upregulate EGF [136]. However as with a number of other cytokines it is also proinflammatory increasing the risk of wound vascularisation [123], neutrophil mediated tissue damage [142] and recurrent erosions [241]. IL–6 also has been shown to promote cell migration [214,386] and as with PDGF–BB this appears to be dependent on the presence of fibronectin [135]. None of the signalling molecules have been clinically trialled, presumably due to the anticipated inflammatory adverse events (Table 1).
Lipids
The lipids that have been shown to promote corneal epithelial wound healing [258,259] are part of the arachidonic acid cascade. These metabolites all promote their effect by enhanced cell migration [259]. However as part of an inflammatory pathway they also recruit high levels of PMNs into the corneal, risking secondary tissue damage [258]. These studies have however helped demonstrate that metabolites of inflammatory pathways such as HETE [259], lipoxin A4 and NPD1 [258] contribute to the epithelial healing process (Table 1).
Proteases
Proteases, in particular uPA and MMP–9, are essential for normal corneal epithelial wound healing [387,388]. Investigations have shown that these enzymes become activated in migrating corneal epithelium and their inhibition results in retarded healing in the case of uPA and abnormal basement membrane formation in the case of MMP–9 [192,389]. Conversely delayed wound healing is often characterised by persistence of these proteases and as such the focus on proteases in the promotion of wound healing has been to limit their activity [390,391]. Oral tetracycline has been used to inhibit MMPs [267] that reduces the recurrence of erosions and promotes epithelisation of corneal erosions, presumably aiding the attachment phase [268]. An animal model has also been used to show it is of benefit in epithelialising alkali burns [270]. By contrast uPA release in response to annexin–A5 has been shown to promote corneal wound healing in vivo for chemical and mechanical debridement models by increasing migration rates [263]. However none of the proteases have been used clinically due to concerns of inducing corneal perforation (Table 1) [392].
Cationic Peptides
The only cationic peptide to be trialled clinically for reepithelisation is a tetra–peptide from the C domain of IGF that is used in conjunction with peptides from carboxyl–terminal of substance P and has been shown to promote wound healing [273]. In this case series, 19 of 26 patients with neurotrophic ulcers unresponsive to standard therapy were successfully treated by co-administration of these peptides [273]. The IGF peptide does not interact with any known IGF receptors and does not stimulate cell proliferation or neovascularisation [393] as is observed for the whole IGF molecule [366]. Another wound healing cationic tetra–peptide, derived from IL–1, also seems to not interact with known IL–1 receptors as it has none of the native–IL–1 proinflammatory properties [272]. Given that the terminal amino acid of both peptides is cationic and the other three amino acids are neural [272,393] it may be the wound healing effects are purely charge based interactions. The cationic antimicrobial protein 37, azurocidin, has also been shown to promote corneal epithelial wound healing in vitro [278]. With no mechanism identified this peptide may also derive its healing property from the positively charged amino acids within the N–terminal peptide sequence. It is less certain if the cationic terminals are directly responsible for the healing properties of LL–37 and the carboxyl tri–peptide sequence of α–melanocyte stimulating hormone as they have been shown to (respectively) increase levels of HB–EGF [275] or nitric oxide [280], both of which have been identified as promoters of corneal epithelial wound healing (Table 1).
Antioxidants
In the context of corneal wound healing antioxidants are primarily used in the management of alkali burns. Vitamin C, ascorbate, helps reverse the scorbutic state of the stroma that follows alkali injury thus minimising the loss of GAGs [283]. This supports the production of collagen type I and collagen type III by keratinocytes,[394] creating a favourable environment for the restoration of the stromal matrix over which the epithelium can reform [367]. Topical ascorbate is usually co–administered with topical citrate which chelates divalent cations, inhibiting MMPs and PMN infiltration [284] supporting reepithelialisation[ 285]. A review of hospital records suggests that this dual therapy is of benefit in severe cases but healing is delayed by 2 days when used in moderate cases [11]. There have also been small RCTs on the use of N-acetylcysteine and combined vitamin A and vitamin E for recovery from refractive surgery. While the benefit of N-acetylcysteine appears to be mostly reduced inflammation [286], high dose vitamins A and E were able to accelerate re–epithelialisation (Table 1) [285].
Proteins, Glycoproteins, Glycosaminoglycans and Saccharides
It has been demonstrated that during the epithelial healing process there is a significant increase in the rate of protein, in particular glycoprotein, synthesis [84]. Blocking the synthesis of protein or asparagine–linked glycoproteins inhibits cell migration [103,395]. Furthermore, the use of lectins to block sugar chains on glycoproteins and GAGs has been shown to inhibit cell migration in debrided ex vivo corneas [374]. This suggests an important role for elevated levels of existing, or new, proteins and glycoproteins in cell migration during wound healing. Galectin–3 and galectin–7 have been shown to promote cell migration in ex vivo corneal alkali burns [340] while lectin KM+ promotes wound healing in vivo in a debridement model, possibly through induction of a mild inflammatory response [372,373]. Despite this evidence for an important role of glycoproteins in wound healing none have been clearly proven to have good clinical efficacy. The agent best supported by a number of case series as a treatment for persistent epithelial defects is fibronectin [352,369] with in vitro data suggesting it promotes adhesion and migration of corneal epithelial cells [339]. However two RCTs did not find any benefit over the control [345,349]. Also tested in RCTs but found to be of no additional benefit in wound healing was hyaluronan [341,371] and a cellulose–based polysaccharide [353]. Other agents such as lactoferrin [338], vitronectin [370] and pigment epithelialderived factor [346] show promise in animal models but are yet to be tested clinically (Table 1).
Anti–inflammatories
Wound healing is normally accompanied by inflammation that is triggered by the inciting injury [134,396]. In fact it appears that some level of inflammation is required for wound healing as disruption of key inflammatory pathways can interrupt the wound closure process [397]. Conversely excess levels of inflammation are believed to be a major contributing factor in the delayed healing of corneal wounds caused alkali burns [285]. The use of COX inhibitors to block production of prostaglandins that recruit neutrophils into the cornea [355] have been investigated in several RCTs on treatments for corneal abrasions. These report that while COX inhibitors improve symptoms they do not actually accelerate the healing process [377]. Control of inflammation with glucocorticosteroids across a wide range (Table 1) of corneal lesions has been investigated in RCTs however in terms of wound healing they generally have no significant impact on re–epithelialisation times [359,364,379] although excess use can delay would closure [378,398]. Thymosin β–4 has been used successfully in two case series of neurotrophic ulcers unresponsive to conventional therapy [375,376] but it has yet to be tested in a RCT. Honey has also had limited success in animal models but it has yet to be tested clinically (Table 1) [380].
Medical Devices and Surgery
Pressure patching has a long history as a management strategy for corneal abrasions. This practice is driven by the belief that the combination a closed eye environment and removal of friction from the lids during the blink improves comfort and promotes wound healing [350]. However a meta–analysis of RCTs for patching of abrasions found there is no increase in healing rates [358]. A similar philosophy also underpins the use of bandage contact lenses, the use of which is not supported by the bulk of literature [342,344,361,362,381] although a modified bandage contact lens called PROSE and the medical or surgical forced closure of the lid have shown promise in case series [351,360,382] however neither has been tested in an RCT. The final iteration in this protective barrier theory is the collagen shield that has been shown in an RCT to promote re–epithelialisation [383] but the mechanism may involve it acting as a sacrificial substrate from MMPs [343] rather than as a barrier. Debridement is a technique commonly used in poorly healing wounds however the benefits of its use have not been well studied; the only trial that reports healing times found no significant difference [268] however there is some evidence it can reduce recurrence rates [363] indicating better quality of healing during the attachment phase. For patients with corneal limbal stem cell deficiency it has recently been demonstrated in a small case series that autologous progenitor stem cells can be transplanted onto the cornea after being cultured onto a bandage contact lens to promote reepithelialisation [384]. The authors propose this creates a new stem cell population that is able to proliferate and repopulate the cornea. A more invasive procedure that is supported by RCTs is attachment of amniotic membranes to promote wound healing in ocular burns and neurotrophic ulcers [347,356]. These promote re-epithelialisation, reduce inflammation and limiting scar formation [354] by acting as a source of growth factors [348]. The most invasive procedure is the corneal graft which only achieves a visual acuity of 6⁄18 or better in approximately 60% of cases (Table 1) [357].
Conclusion
Whilst corneal wounding is common, it does not always result in major sequelae or effects on vision. However, under circumstances such as traumatic eye injury involving alkaline agents or infection, healing is compromised and may result in loss of vision. For these reasons several therapeutic agents can be used to facilitate wound healing, and several new types are being actively investigated in laboratory models. Having the current and new armamentarium will hopefully result in improved outcomes for patients and reduced costs associated with treatment.
Acknowledgments
This work was supported by a grant from Dairy Australia.
References
- Nash EA, Margo CE. Patterns of emergency department visits for disorders of the eye and ocular adnexa. Arch Ophthalmol. 1998; 116: 1222-1226.
- Fea A, Bosone A, Rolle T, Grignolo FM. Eye injuries in an Italian urban population: report of 10,620 cases admitted to an eye emergency department in Torino. Graefes Arch Clin Exp Ophthalmol. 2008; 246: 175-179.
- Mcgwin G, Jr., Owsley C. Incidence of emergency department-treated eye injury in the United States. Arch Ophthalmol. 2005; 123: 662-666.
- Karlson TA, Klein BE. The incidence of acute hospital-treated eye injuries. Arch Ophthalmol. 1986; 104: 1473-1476.
- Katz J, Tielsch JM. Lifetime prevalence of ocular injuries from the Baltimore Eye Survey. Arch Ophthalmol. 1993; 111: 1564-1568.
- Mccarty CA, Fu CL, Taylor HR. Epidemiology of ocular trauma in Australia. Ophthalmology. 1999; 106: 1847-1852.
- Wang JD, Xu L, Wang YX, You QS, Zhang JS et al. Prevalence and incidence of ocular trauma in North China: the Beijing Eye Study. Acta Ophthalmol. 2011.
- Khatry SK, Lewis AE, Schein OD, Thapa MD, Pradhan EK, et al. The epidemiology of ocular trauma in rural Nepal. Br J Ophthalmol2004; 88: 456-460.
- Morgan SJ. Chemical burns of the eye: causes and management. Br J Ophthalmol. 1987; 71: 854-857.
- Loon SC, Tay WT, Saw SM, Wang JJ, Wong TY. Prevalence and risk factors of ocular trauma in an urban south-east Asian population: the Singapore Malay Eye Study. Clin Experiment Ophthalmol. 2009; 37: 362-367.
- Brodovsky SC, Mccarty CA, Snibson G, Loughnan M, Sullivan L et al. Management of alkali burns : an 11-year retrospective review. Ophthalmology. 2000; 107: 1829-1835.
- Moon ME, Robertson IF. Retrospective study of alkali burns of the eye. Aust J Ophthalmol. 1983; 11: 281-286.
- Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008; 27: 22-27.
- Stapleton F, Keay L, Edwards K, Naduvilath T, Dart JK, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008; 115: 1655-1662.
- Poggio EC, Glynn RJ, Schein OD, Seddon JM, Shannon MJ et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989; 321: 779-783.
- Lam DS, Houang E, Fan DS, Lyon D, Seal D, et al. Incidence and risk factors for microbial keratitis in Hong Kong: comparison with Europe and North America. Eye (Lond). 2002; 16: 608-618.
- Cheng KH, Leung SL, Hoekman HW, Beekhuis WH, Mulder PG et al. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999; 354: 181-185.
- Erie JC, Nevitt MP, Hodge DO, Ballard DJ. Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch Ophthalmol. 1993; 111: 1665-1671.
- Gonzales CA, Srinivasan M, Whitcher JP, Smolin G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol. 1996; 3: 159-166.
- Bailey MD, Zadnik K. Outcomes of LASIK for myopia with FDA-approved lasers. Cornea. 2007; 26: 246-254.
- Leaming DV. Practice styles and preferences of ASCRS members--2003 survey. J Cataract Refract Surg. 2004; 30: 892-900.
- Kanthan GL, Wang JJ, Rochtchina E, Tan AG, Lee A, et al. Ten-year incidence of age-related cataract and cataract surgery in an older Australian population. The Blue Mountains Eye Study. Ophthalmology. 2008; 115: 808-814 e801.
- Alexander DA, Kemp RV, Klein S, Forrester JV. Psychiatric sequelae and psychosocial adjustment following ocular trauma: a retrospective pilot study. Br J Ophthalmol. 2001; 85: 560-562.
- Goyal R, Shankar J, Fone DL, Hughes DS. Randomised controlled trial of ketorolac in the management of corneal abrasions. Acta Ophthalmol Scand. 2001; 79: 177-179.
- Meek R, Sullivan A, Favilla M, Larmour I, Guastalegname S. Is homatropine 5% effective in reducing pain associated with corneal abrasion when compared with placebo? A randomized controlled trial. Emerg Med Australas. 2010; 22: 507-513.
- Patterson J, Fetzer D, Krall J, Wright E, Heller M. Eye patch treatment for the pain of corneal abrasion. South Med J. 1996; 89: 227-229.
- Kaiser PK. A comparison of pressure patching versus no patching for corneal abrasions due to trauma or foreign body removal. Corneal Abrasion Patching Study Group. Ophthalmology. 1995; 102: 1936-1942.
- Klein R, Lobes LA, Jr. Ocular alkali burns in a large urban area. Ann Ophthalmol. 1976; 8: 1185-1189.
- Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001; 79: 214-221.
- Keay L, Edwards K, Naduvilath T, Forde K, Stapleton F. Factors affecting the morbidity of contact lens-related microbial keratitis: a population study. Invest Ophthalmol Vis Sci. 2006; 47: 4302-4308.
- Prajna VN, Nirmalan PK, Saravanan S, Srinivasan M. Economic analysis of corneal ulcers in South India. Cornea. 2007; 26: 119-122.
- Keay L, Edwards K, Dart J, Stapleton F. Grading contact lens-related microbial keratitis: relevance to disease burden. Optom Vis Sci. 2008; 85: 531-537.
- Wong T, Ormonde S, Gamble G, Mcghee CN. Severe infective keratitis leading to hospital admission in New Zealand. Br J Ophthalmol. 2003; 87: 1103-1108.
- Stein JD, Grossman DS, Mundy KM, Sugar A, Sloan FA. Severe adverse events after cataract surgery among medicare beneficiaries. Ophthalmology. 2011; 118: 1716-1723.
- Nichamin LD, Chang DF, Johnson SH, Mamalis N, Masket S et al. ASCRS White Paper: What is the association between clear corneal cataract incisions and postoperative endophthalmitis? J Cataract Refract Surg. 2006; 32: 1556-1559.
- Taban M, Behrens A, Newcomb RL, Nobe MY, Saedi G, et al. Acute endophthalmitis following cataract surgery: a systematic review of the literature. Arch Ophthalmol. 2005; 123: 613-620.
- Sugar A, Rapuano CJ, Culbertson WW, Huang D, Varley GA et al. Laser in situ keratomileusis for myopia and astigmatism: safety and efficacy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002; 109: 175-187.
- El Danasoury MA, El Maghraby A, Klyce SD, Mehrez K. Comparison of photorefractive keratectomy with excimer laser in situ keratomileusis in correcting low myopia (from -2.00 to -5.50 diopters). A randomized study. Ophthalmology. 1999; 106: 411-420; discussion 420-411.
- Ghadhfan F, Al-Rajhi A, Wagoner MD. Laser in situ keratomileusis versus surface ablation: visual outcomes and complications. J Cataract Refract Surg. 2007; 33: 2041-2048.
- Shortt AJ, Allan BD. Photorefractive keratectomy (PRK) versus laser-assisted in-situ keratomileusis (LASIK) for myopia. Cochrane Database Syst Rev. 2006: CD005135.
- Delmonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011; 37: 588-598.
- Van Buskirk EM. The anatomy of the limbus. Eye (Lond). 1989; 3 ( Pt 2): 101-108.
- Holly FJ. Tear film physiology. Int Ophthalmol Clin. 1987; 27: 2-6.
- Rolando M, Zierhut M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol. 2001; 45 Suppl 2: S203-210.
- Paulsen F. Functional anatomy and immunological interactions of ocular surface and adnexa. Dev Ophthalmol. 2008; 41: 21-35.
- Suzuki K, Saito J, Yanai R, Yamada N, Chikama T et al. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Prog Retin Eye Res. 2003; 22: 113-133.
- Beuerman RW, Pedroza L. Ultrastructure of the human cornea. Microsc Res Tech. 1996; 33: 320-335.
- Hanna C, Bicknell DS, O'brien JE. Cell turnover in the adult human eye. Arch Ophthalmol. 1961; 65: 695-698.
- Ban Y, Dota A, Cooper LJ, Fullwood NJ, Nakamura T et al. Tight junction-related protein expression and distribution in human corneal epithelium. Exp Eye Res. 2003; 76: 663-669.
- Tonjum AM. Permeability of horseradish peroxidase in the rabbit corneal epithelium. Acta Ophthalmol (Copenh). 1974; 52: 650-658.
- Suzuki K, Tanaka T, Enoki M, Nishida T. Coordinated reassembly of the basement membrane and junctional proteins during corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2000; 41: 2495-2500.
- Mclaughlin BJ, Caldwell RB, Sasaki Y, Wood TO. Freeze-fracture quantitative comparison of rabbit corneal epithelial and endothelial membranes. Curr Eye Res. 1985; 4: 951-961.
- Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011; 192: 907-917.
- Lavker RM, Dong G, Cheng SZ, Kudoh K, Cotsarelis G et al. Relative proliferative rates of limbal and corneal epithelia. Implications of corneal epithelial migration, circadian rhythm, and suprabasally located DNA-synthesizing keratinocytes. Invest Ophthalmol Vis Sci. 1991; 32: 1864-1875.
- Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983; 24: 1442-1443.
- Simpson I, Rose B, Loewenstein WR. Size limit of molecules permeating the junctional membrane channels. Science. 1977; 195: 294-296.
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996; 84: 381-388.
- Stepp MA, Spurr-Michaud S, Tisdale A, Elwell J, Gipson IK. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A. 1990; 87: 8970-8974.
- Gipson IK, Spurr-Michaud SJ, Tisdale AS. Anchoring fibrils form a complex network in human and rabbit cornea. Invest Ophthalmol Vis Sci. 1987; 28: 212-220.
- Stepp MA. Corneal integrins and their functions. Exp Eye Res. 2006; 83: 3-15.
- Alvarado J, Murphy C, Juster R. Age-related changes in the basement membrane of the human corneal epithelium. Invest Ophthalmol Vis Sci. 1983; 24: 1015-1028.
- Yurchenco PD, Schittny JC. Molecular architecture of basement membranes. Faseb J. 1990; 4: 1577-1590.
- Sakai LY, Keene DR, Morris NP, Burgeson RE. Type VII collagen is a major structural component of anchoring fibrils. J Cell Biol. 1986; 103: 1577-1586.
- Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991; 114: 567-576.
- Knupp C, Pinali C, Lewis PN, Parfitt GJ, Young RD et al. The architecture of the cornea and structural basis of its transparency. Adv Protein Chem Struct Biol. 2009; 78: 25-49.
- Birk DE, Fitch JM, Linsenmayer TF. Organization of collagen types I and V in the embryonic chicken cornea. Invest Ophthalmol Vis Sci. 1986; 27: 1470-1477.
- Nakayasu K, Tanaka M, Konomi H, Hayashi T. Distribution of types I, II, III, IV and V collagen in normal and keratoconus corneas. Ophthalmic Res. 1986; 18: 1-10.
- Delaigue O, Arbeille B, Rossazza C, Lemesle M, Roingeard P. Quantitative analysis of immunogold labellings of collagen types I, III, IV and VI in healthy and pathological human corneas. Graefes Arch Clin Exp Ophthalmol. 1995; 233: 331-338.
- White J, Werkmeister JA, Ramshaw JA, Birk DE. Organization of fibrillar collagen in the human and bovine cornea: collagen types V and III. Connect Tissue Res. 1997; 36: 165-174.
- Edelhauser HF. The balance between corneal transparency and edema: the Proctor Lecture. Invest Ophthalmol Vis Sci. 2006; 47: 1754-1767.
- Jue B, Maurice DM. The mechanical properties of the rabbit and human cornea. J Biomech. 1986; 19: 847-853.
- Tao A, Wang J, Chen Q, Shen M, Lu F et al. Topographic thickness of Bowman's layer determined by ultra-high resolution spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 3901-3907.
- West-Mays JA, Dwivedi DJ. The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol. 2006; 38: 1625-1631.
- Muller LJ, Pels L, Vrensen GF. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995; 36: 2557-2567.
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT et al. The cellular basis of corneal transparency: evidence for 'corneal crystallins'. J Cell Sci. 1999; 112 ( Pt 5): 613-622.
- Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003; 22: 359-389.
- Bonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res. 2011.
- Tuft SJ, Coster DJ. The corneal endothelium. Eye (Lond). 1990; 4: 389-424.
- Pfister RR, Burstein N. The alkali burned cornea I. Epithelial and stromal repair. Exp Eye Res. 1976; 23: 519-535.
- Allen VD, Malinovsky V. Management of neurotrophic keratopathy. Cont Lens Anterior Eye. 2003; 26: 161-165.
- Bikbova G, Oshitari T, Tawada A, Yamamoto S. Corneal changes in diabetes mellitus. Curr Diabetes Rev. 2012; 8: 294-302.
- Agrawal VB, Tsai RJ. Corneal epithelial wound healing. Indian J Ophthalmol. 2003; 51: 5-15.
- Crosson CE, Klyce SD, Beuerman RW. Epithelial wound closure in the rabbit cornea. A biphasic process. Invest Ophthalmol Vis Sci. 1986; 27: 464-473.
- Zieske JD, Gipson IK. Protein synthesis during corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 1986; 27: 1-7.
- Kuwabara T, Perkins DG, Cogan DG. Sliding of the epithelium in experimental corneal wounds. Invest Ophthalmol. 1976; 15: 4-14.
- Hanna C. Proliferation and migration of epithelial cells during corneal wound repair in the rabbit and the rat. Am J Ophthalmol. 1966; 61: 55-63.
- Gipson IK, Spurr-Michaud S, Tisdale A, Keough M. Reassembly of the anchoring structures of the corneal epithelium during wound repair in the rabbit. Invest Ophthalmol Vis Sci. 1989; 30: 425-434.
- Estil S, Kravik K, Haaskjold E, Refsum SB, Bjerknes R et al. Pilot study on the time course of apoptosis in the regenerating corneal epithelium. Acta Ophthalmol Scand. 2002; 80: 517-523.
- Fujikawa LS, Foster CS, Harrist TJ, Lanigan JM, Colvin RB. Fibronectin in healing rabbit corneal wounds. Lab Invest. 1981; 45: 120-129.
- Buck RC. Hemidesmosomes of normal and regenerating mouse corneal epithelium. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982; 41: 1-16.
- Khodadoust AA, Silverstein AM, Kenyon DR, Dowling JE. Adhesion of regenerating corneal epithelium. The role of basement membrane. Am J Ophthalmol. 1968; 65: 339-348.
- Gipson IK, Spurr-Michaud S, Tisdale A, Elwell J, Stepp MA. Redistribution of the hemidesmosome components alpha 6 beta 4 integrin and bullous pemphigoid antigens during epithelial wound healing. Exp Cell Res. 1993; 207: 86-98.
- Murakami J, Nishida T, Otori T. Coordinated appearance of beta 1 integrins and fibronectin during corneal wound healing. J Lab Clin Med. 1992; 120: 86-93.
- Blanco-Mezquita JT, Hutcheon AE, Stepp MA, Zieske JD. alphaVbeta6 integrin promotes corneal wound healing. Invest Ophthalmol Vis Sci. 2011; 52: 8505-8513.
- Soong HK. Vinculin in focal cell-to-substrate attachments of spreading corneal epithelial cells. Arch Ophthalmol. 1987; 105: 1129-1132.
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992; 69: 11-25.
- Lazarides E. Immunofluorescence studies on the structure of actin filaments in tissue culture cells. J Histochem Cytochem. 1975; 23: 507-528.
- Yamada M, Mashima Y, Tsubota K. Scanning electron microscopic observation of basal cells following corneal epithelial abrasion. Eye (Lond). 1996; 10: 569-574.
- Anderson RA. Actin filaments in normal and migrating corneal epithelial cells. Invest Ophthalmol Vis Sci. 1977; 16: 161-166.
- Singer, Ii. The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell. 1979; 16: 675-685.
- Matsuda M, Ubels JL, Edelhauser HF. A larger corneal epithelial wound closes at a faster rate. Invest Ophthalmol Vis Sci. 1985; 26: 897-900.
- Frati L, Daniele S, Delogu A, Covelli I. Selective binding of the epidermal growth factor and its specific effects on the epithelial cells of the cornea. Exp Eye Res. 1972; 14: 135-141.
- Gibbins JR. Epithelial migration in organ culture. Role of protein synthesis as determined by metabolic inhibitors. Exp Cell Res. 1973; 80: 281-290.
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989; 57: 201-209.
- Lehrer MS, Sun TT, Lavker RM. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci. 1998; 111: 2867-2875.
- Chung EH, Hutcheon AE, Joyce NC, Zieske JD. Synchronization of the G1/S transition in response to corneal debridement. Invest Ophthalmol Vis Sci. 1999; 40: 1952-1958.
- Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood). 2001; 226: 653-664.
- Mccartney MD, Cantu-Crouch D. Rabbit corneal epithelial wound repair: tight junction reformation. Curr Eye Res. 1992; 11: 15-24.
- Gipson IK. Adhesive mechanisms of the corneal epithelium. Acta Ophthalmol Suppl. 1992: 13-17.
- Gipson IK, Spurr-Michaud SJ, Tisdale AS. Hemidesmosomes and anchoring fibril collagen appear synchronously during development and wound healing. Dev Biol. 1988; 126: 253-262.
- Fini ME, Stramer BM. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005; 24: S2-S11.
- Wilson SE, Kim WJ. Keratocyte apoptosis: implications on corneal wound healing, tissue organization, and disease. Invest Ophthalmol Vis Sci. 1998; 39: 220-226.
- Robb RM, Kuwabara T. Corneal wound healing. I. The movement of polymorphonuclear leukocytes into corneal wounds. Arch Ophthalmol. 1962; 68: 636-642.
- Kenyon KR. Inflammatory mechanisms in corneal ulceration. Trans Am Ophthalmol Soc. 1985; 83: 610-663.
- #115. Gan L, Fagerholm P, Kim HJ. Effect of leukocytes on corneal cellular proliferation and wound healing. Invest Ophthalmol Vis Sci. 1999; 40: 575-581.
- Wilson SE, He YG, Weng J, Li Q, Mcdowall AW et al. Epithelial injury induces keratocyte apoptosis: Hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Experimental Eye Research. 1996; 62: 325-337.
- Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999; 18: 529-551.
- Malecaze F, Simorre V, Chollet P, Tack JL, Muraine M et al. Interleukin-6 in tear fluid after photorefractive keratectomy and its effects on keratocytes in culture. Cornea. 1997; 16: 580-587.
- Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005; 13: 7-12
- Matsubara M, Girard MT, Kublin CL, Cintron C, Fini ME. Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodelling cornea. Dev Biol. 1991; 147: 425-439.
- Strissel KJ, Rinehart WB, Fini ME. Regulation of paracrine cytokine balance controlling collagenase synthesis by corneal cells. Invest Ophthalmol Vis Sci. 1997; 38: 546-552.
- Gabison EE, Huet E, Baudouin C, Menashi S. Direct epithelial-stromal interaction in corneal wound healing: Role of EMMPRIN/CD147 in MMPs induction and beyond. Prog Retin Eye Res. 2009; 28: 19-33.
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, Dipietro LA et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992; 258: 1798-1801.
- Yee RW, Geroski DH, Matsuda M, Champeau EJ, Meyer LA et al. Correlation of corneal endothelial pump site density, barrier function, and morphology in wound repair. Invest Ophthalmol Vis Sci. 1985; 26: 1191-1201.
- Faure JP, Yong ZK. [Observations on the effects of alloxan on the cornea of the guinea pig. I. Destruction and regeneration of the endothelium]. Arch Ophtalmol Rev Gen Ophtalmol. 1966; 26: 677-686.
- Van Horn DL, Sendele DD, Seideman S, Buco PJ. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest Ophthalmol Vis Sci. 1977; 16: 597-613.
- Matsuda M, Suda T, Manabe R. Serial alterations in endothelial cell shape and pattern after intraocular surgery. Am J Ophthalmol. 1984; 98: 313-319.
- Foster CS, Zelt RP, Mai-Phan T, Kenyon KR. Immunosuppression and selective inflammatory cell depletion. Studies on a guinea pig model of corneal ulceration after ocular alkali burning. Arch Ophthalmol. 1982; 100: 1820-1824.
- Khodadoust AA, Green K. Physiological function of regenerating endothelium. Invest Ophthalmol. 1976; 15: 96-101.
- Chi HH, Kelman CD. Effects of freezing on ocular tissues. I. Clinical and histologic study of corneal endothelium. Am J Ophthalmol. 1966; 61: 630-641.
- Morton PL, Ormsby HL, Basu PK. Healing of endothelium and Descemet's membrane of rabbit cornea. Am J Ophthalmol. 1958; 46: 62-67.
- Yu FS, Yin J, Xu K, Huang J. Growth factors and corneal epithelial wound healing. Brain Res Bull. 2010; 81: 229-235.
- Chandrasekher G, Bazan HE. Corneal epithelial wound healing increases the expression but not long lasting activation of the p85alpha subunit of phosphatidylinositol-3 kinase. Curr Eye Res. 1999; 18: 168-176.
- Sotozono C, He J, Matsumoto Y, Kita M, Imanishi J et al. Cytokine expression in the alkali-burned cornea. Curr Eye Res. 1997; 16: 670-676.
- Nishida T, Nakamura M, Mishima H, Otori T. Interleukin 6 promotes epithelial migration by a fibronectin-dependent mechanism. J Cell Physiol. 1992; 153: 1-5.
- Boisjoly HM, Laplante C, Bernatchez SF, Salesse C, Giasson M et al. Effects of EGF, IL-1 and their combination on in vitro corneal epithelial wound closure and cell chemotaxis. Exp Eye Res. 1993; 57: 293-300.
- Weng J, Mohan RR, Li Q, Wilson SE. IL-1 upregulates keratinocyte growth factor and hepatocyte growth factor mRNA and protein production by cultured stromal fibroblast cells: interleukin-1 beta expression in the cornea. Cornea. 1997; 16: 465-471.
- Girard MT, Matsubara M, Fini ME. Transforming growth factor-beta and interleukin-1 modulate metalloproteinase expression by corneal stromal cells. Invest Ophthalmol Vis Sci. 1991; 32: 2441-2454.
- Stapleton WM, Chaurasia SS, Medeiros FW, Mohan RR, Sinha S et al. Topical interleukin-1 receptor antagonist inhibits inflammatory cell infiltration into the cornea. Exp Eye Res. 2008; 86: 753-757.
- Mohan RR, Liang Q, Kim WJ, Helena MC, Baerveldt F et al. Apoptosis in the cornea: Further characterization of Fas/Fas ligand system. Experimental Eye Research. 1997; 65: 575-589.
- Cubitt CL, Tang Q, Monteiro CA, Lausch RN, Oakes JE. IL-8 gene expression in cultures of human corneal epithelial cells and keratocytes. Invest Ophthalmol Vis Sci. 1993; 34: 3199-3206.
- Mukaida N, Harada A, Yasumoto K, Matsushima K. Properties of pro-inflammatory cell type-specific leukocyte chemotactic cytokines, interleukin 8 (IL-8) and monocyte chemotactic and activating factor (MCAF). Microbiol Immunol. 1992; 36: 773-789.
- Saika S. Yin and Yang in cytokine regulation of corneal wound healing: Roles of TNF-a. Cornea. 2007; 26.
- Mohan RR, Kim WJ, Wilson SE. Modulation of TNF-alpha-induced apoptosis in corneal fibroblasts by transcription factor NF-kappaB. Invest Ophthalmol Vis Sci. 2000; 41: 1327-1336.
- Elner VM, Strieter RM, Pavilack MA, Elner SG, Remick DG et al. Human corneal interleukin-8. IL-1 and TNF-induced gene expression and secretion. Am J Pathol. 1991; 139: 977-988.
- Wilson SE, Mohan RR, Ambrã³sio Jr R, Hong J, Lee J. The corneal wound healing response: Cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Progress in Retinal and Eye Research. 2001; 20: 625-637.
- Wilson SE, He YG, Weng J, Zieske JD, Jester JV et al. Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp Eye Res. 1994; 59: 665-678.
- Wilson SE, Chen L, Mohan RR, Liang Q, Liu J. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp Eye Res. 1999; 68: 377-397.
- Li DQ, Tseng SC. Differential regulation of keratinocyte growth factor and hepatocyte growth factor/scatter factor by different cytokines in human corneal and limbal fibroblasts. J Cell Physiol. 1997; 172: 361-372.
- Wilson SE, Walker JW, Chwang EL, He YG. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Invest Ophthalmol Vis Sci. 1993; 34: 2544-2561.
- Lambiase A, Manni L, Bonini S, Rama P, Micera A et al. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci. 2000; 41: 1063-1069.
- Kruse FE, Tseng SC. Growth factors modulate clonal growth and differentiation of cultured rabbit limbal and corneal epithelium. Invest Ophthalmol Vis Sci. 1993; 34: 1963-1976.
- Wilson SE, Liang Q, Kim WJ. Lacrimal gland HGF, KGF, and EGF mRNA levels increase after corneal epithelial wounding. Invest Ophthalmol Vis Sci. 1999; 40: 2185-2190.
- Kitazawa T, Kinoshita S, Fujita K, Araki K, Watanabe H et al. The mechanism of accelerated corneal epithelial healing by human epidermal growth factor. Invest Ophthalmol Vis Sci. 1990; 31: 1773-1778.
- Zieske JD, Takahashi H, Hutcheon AE, Dalbone AC. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest Ophthalmol Vis Sci. 2000; 41: 1346-1355.
- Tuli SS, Liu R, Chen C, Blalock TD, Goldstein M et al. Immunohistochemical localization of EGF, TGF-alpha, TGF-beta, and their receptors in rat corneas during healing of excimer laser ablation. Curr Eye Res. 2006; 31: 709-719.
- Denk PO, Knorr M. The in vitro effect of platelet-derived growth factor isoforms on the proliferation of bovine corneal stromal fibroblasts depends on cell density. Graefes Arch Clin Exp Ophthalmol. 1997; 235: 530-534.
- Andresen JL, Ehlers N. Chemotaxis of human keratocytes is increased by platelet-derived growth factor-BB, epidermal growth factor, transforming growth factor-alpha, acidic fibroblast growth factor, insulin-like growth factor-I, and transforming growth factor-beta. Curr Eye Res. 1998; 17: 79-87.
- Kamiyama K, Iguchi I, Wang X, Imanishi J. Effects of PDGF on the migration of rabbit corneal fibroblasts and epithelial cells. Cornea. 1998; 17: 315-325.
- Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996; 15: 505-516.
- Nishida K, Kinoshita S, Yokoi N, Kaneda M, Hashimoto K, et al. Immunohistochemical localization of transforming growth factor-beta 1, -beta 2, and -beta 3 latency-associated peptide in human cornea. Invest Ophthalmol Vis Sci. 1994; 35: 3289-3294.
- Honma Y, Nishida K, Sotozono C, Kinoshita S. Effect of transforming growth factor-beta1 and -beta2 on in vitro rabbit corneal epithelial cell proliferation promoted by epidermal growth factor, keratinocyte growth factor, or hepatocyte growth factor. Exp Eye Res. 1997; 65: 391-396.
- Xu KP, Ding Y, Ling J, Dong Z, Yu FS. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004; 45: 813-820.
- Block ER, Matela AR, Sundarraj N, Iszkula ER, Klarlund JK. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem. 2004; 279: 24307-24312.
- Boucher I, Yang L, Mayo C, Klepeis V, Trinkaus-Randall V. Injury and nucleotides induce phosphorylation of epidermal growth factor receptor: MMP and HB-EGF dependent pathway. Exp Eye Res. 2007; 85: 130-141.
- Xu KP, Riggs A, Ding Y, Yu FS. Role of ErbB2 in Corneal Epithelial Wound Healing. Invest Ophthalmol Vis Sci. 2004; 45: 4277-4283.
- Yin J, Yu FS. Rho kinases regulate corneal epithelial wound healing. Am J Physiol Cell Physiol. 2008; 295: C378-387.
- Zhang Y, Akhtar RA. Epidermal growth factor stimulation of phosphatidylinositol 3-kinase during wound closure in rabbit corneal epithelial cells. Invest Ophthalmol Vis Sci. 1997; 38: 1139-1148.
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995; 15: 1942-1952.
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995; 81: 53-62.
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992; 70: 389-399.
- Nakamura M, Nagano T, Chikama T, Nishida T. Role of the small GTP-binding protein rho in epithelial cell migration in the rabbit cornea. Invest Ophthalmol Vis Sci. 2001; 42: 941-947.
- Hall A, Nobes CD. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci. 2000; 355: 965-970.
- Gipson IK, Westcott MJ, Brooksby NG. Effects of cytochalasins B and D and colchicine on migration of the corneal epithelium. Invest Ophthalmol Vis Sci. 1982; 22: 633-642.
- Anderson SC, Stone C, Tkach L, Sundarraj N. Rho and Rho-kinase (ROCK) signaling in adherens and gap junction assembly in corneal epithelium. Invest Ophthalmol Vis Sci. 2002; 43: 978-986.
- Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem. 2003; 278: 21989-21997.
- Chandrasekher G, Kakazu AH, Bazan HE. HGF- and KGF-induced activation of PI-3K/p70 s6 kinase pathway in corneal epithelial cells: its relevance in wound healing. Exp Eye Res. 2001; 73: 191-202.
- Sharma GD, Kakazu A, Bazan HE. Protein kinase C alpha and epsilon differentially modulate hepatocyte growth factor-induced epithelial proliferation and migration. Exp Eye Res. 2007; 85: 289-297.
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003; 76: 521-542.
- Garcia-Hirschfeld J, Lopez-Briones LG, Belmonte C. Neurotrophic influences on corneal epithelial cells. Exp Eye Res. 1994; 59: 597-605.
- Chan KY, Haschke RH. Isolation and culture of corneal cells and their interactions with dissociated trigeminal neurons. Exp Eye Res. 1982; 35: 137-156.
- Baker KS, Anderson SC, Romanowski EG, Thoft RA, Sundarraj N. Trigeminal ganglion neurons affect corneal epithelial phenotype. Influence on type VII collagen expression in vitro. Invest Ophthalmol Vis Sci. 1993; 34: 137-144.
- Nakamura M, Nishida T, Ofuji K, Reid TW, Mannis MJ et al. Synergistic effect of substance P with epidermal growth factor on epithelial migration in rabbit cornea. Exp Eye Res. 1997; 65: 321-329.
- Nakamura M, Nagano T, Chikama T, Nishida T. Up-regulation of phosphorylation of focal adhesion kinase and paxillin by combination of substance P and IGF-1 in SV-40 transformed human corneal epithelial cells. Biochem Biophys Res Commun. 1998; 242: 16-20.
- Nishida T, Nakamura M, Ofuji K, Reid TW, Mannis MJ et al. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J Cell Physiol. 1996; 169: 159-166.
- Liu GS, Trope GE, Basu PK. Beta adrenoceptors and regenerating corneal epithelium. J Ocul Pharmacol. 1990; 6: 101-112.
- Reidy JJ, Zarzour J, Thompson HW, Beuerman RW. Effect of topical beta blockers on corneal epithelial wound healing in the rabbit. Br J Ophthalmol. 1994; 78: 377-380.
- Er H. The effect of topical parasympathomimetics on corneal epithelial healing in rabbits. Doc Ophthalmol. 1997; 93: 327-335.
- Mindel JS, Mittag TW. Variability of choline acetyltransferase in ocular tissues of rabbits, cats, cattle and humans. Exp Eye Res. 1977; 24: 25-33.
- Huttenlocher A, Lakonishok M, Kinder M, Wu S, Truong T et al. Integrin and cadherin synergy regulates contact inhibition of migration and motile activity. J Cell Biol. 1998; 141: 515-526.
- Lampe PD, Nguyen BP, Gil S, Usui M, Olerud J et al. Cellular interaction of integrin alpha3beta1 with laminin 5 promotes gap junctional communication. J Cell Biol. 1998; 143: 1735-1747.
- Morimoto K, Mishima H, Nishida T, Otori T. Role of urokinase type plasminogen activator (u-PA) in corneal epithelial migration. Thromb Haemost. 1993; 69: 387-391.
- Zieske JD, Bukusoglu G. Effect of protease inhibitors on corneal epithelial migration. Invest Ophthalmol Vis Sci. 1991; 32: 2073-2078.
- Barlati S, Marchina E, Quaranta CA, Vigasio F, Semeraro F. Analysis of fibronectin, plasminogen activators and plasminogen in tear fluid as markers of corneal damage and repair. Exp Eye Res. 1990; 51: 1-9.
- Lijnen HR. Elements of the fibrinolytic system. Ann N Y Acad Sci. 2001; 936: 226-236.
- Bonnefoy A, Legrand C. Proteolysis of subendothelial adhesive glycoproteins (fibronectin, thrombospondin, and von Willebrand factor) by plasmin, leukocyte cathepsin G, and elastase. Thromb Res. 2000; 98: 323-332.
- Berman M, Manseau E, Law M, Aiken D. Ulceration is correlated with degradation of fibrin and fibronectin at the corneal surface. Invest Ophthalmol Vis Sci. 1983; 24: 1358-1366.
- Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997; 91: 917-925.
- Berman MB. Regulation of corneal fibroblast MMP-1 collagenase secretion by plasmin. Cornea. 1993; 12: 420-432.
- Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol. 1990; 110: 1361-1367.
- Matsubara M, Zieske JD, Fini ME. Mechanism of basement membrane dissolution preceding corneal ulceration. Invest Ophthalmol Vis Sci. 1991; 32: 3221-3237.
- Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998; 161: 3340-3346.
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000; 14: 163-176.
- Mohan R, Chintala SK, Jung JC, Villar WV, Mccabe F et al. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem. 2002; 277: 2065-2072.
- Daniels JT, Geerling G, Alexander RA, Murphy G, Khaw PT et al. Temporal and spatial expression of matrix metalloproteinases during wound healing of human corneal tissue. Exp Eye Res. 2003; 77: 653-664.
- Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997; 41: 275-313.
- Soong HK, Cintron C. Disparate effects of calmodulin inhibitors on corneal epithelial migration in rabbit and rat. Ophthalmic Res. 1985; 17: 27-33.
- Pastor JC, Calonge M. Epidermal growth factor and corneal wound healing. A multicenter study. Cornea. 1992; 11: 311-314.
- Singh G, Foster CS. Epidermal growth factor in alkali-burned corneal epithelial wound healing. Am J Ophthalmol. 1987; 103: 802-807.
- Geerling G, Unterlauft JD, Kasper K, Schrader S, Opitz A et al. [Autologous serum and alternative blood products for the treatment of ocular surface disorders]. Ophthalmologe. 2008; 105: 623-631.
- Lekhanont K, Jongkhajornpong P, Choubtum L, Chuckpaiwong V. Topical 100% serum eye drops for treating corneal epithelial defect after ocular surgery. Biomed Res Int. 2013; 2013: 521315.
- Kim KM, Shin YT, Kim HK. Effect of autologous platelet-rich plasma on persistent corneal epithelial defect after infectious keratitis. Jpn J Ophthalmol. 2012; 56: 544-550.
- Saika S. TGF-beta signal transduction in corneal wound healing as a therapeutic target. Cornea. 2004; 23: S25-30.
- Er H, Uzmez E. Effects of transforming growth factor-beta 2, interleukin 6 and fibronectin on corneal epithelial wound healing. Eur J Ophthalmol. 1998; 8: 224-229.
- Sakamoto T, Ueno H, Sonoda K, Hisatomi T, Shimizu K et al. Blockade of TGF-beta by in vivo gene transfer of a soluble TGF-beta type II receptor in the muscle inhibits corneal opacification, edema and angiogenesis. Gene Ther. 2000; 7: 1915-1924.
- Liu L, Li YP, Huang SQ, Lin JX, Zhang WX. [Mechanism of keratinocyte growth factor-2 accelerating corneal epithelial wound healing on rabbit alkali burned cornea]. Zhonghua Yan Ke Za Zhi. 2005; 41: 364-368.
- Sotozono C, Inatomi T, Nakamura M, Kinoshita S. Keratinocyte growth factor accelerates corneal epithelial wound healing in vivo. Invest Ophthalmol Vis Sci. 1995; 36: 1524-1529.
- Liu L, Li Y, Huang S, Lin J, Zhang W. Keratinocyte growth factor-2 on the proliferation of corneal epithelial stem cells in rabbit alkali burned cornea. Yan Ke Xue Bao. 2007; 23: 107-116.
- Mishima H, Nakamura M, Murakami J, Nishida T, Otori T. Transforming growth factor-beta modulates effects of epidermal growth factor on corneal epithelial cells. Curr Eye Res. 1992; 11: 691-696.
- Gospodarowicz D, Mescher AL, Brown KD, Birdwell CR. The role of fibroblast growth factor and epidermal growth factorin the proliferative response of the corneal and lens epithelium. Exp Eye Res. 1977; 25: 631-649.
- Grant MB, Khaw PT, Schultz GS, Adams JL, Shimizu RW. Effects of epidermal growth factor, fibroblast growth factor, and transforming growth factor-beta on corneal cell chemotaxis. Invest Ophthalmol Vis Sci. 1992; 33: 3292-3301.
- Rajan MS, Shafiei S, Mohrenfels CV, Patmore A, Lohmann C et al. Effect of exogenous keratinocyte growth factor on corneal epithelial migration after photorefractive keratectomy. J Cataract Refract Surg. 2004; 30: 2200-2206.
- Fredj-Reygrobellet D, Plouet J, Delayre T, Baudouin C, Bourret F et al. Effects of aFGF and bFGF on wound healing in rabbit corneas. Curr Eye Res. 1987; 6: 1205-1209.
- Rieck P, Assouline M, Savoldelli M, Hartmann C, Jacob C et al. Recombinant human basic fibroblast growth factor (Rh-bFGF) in three different wound models in rabbits: corneal wound healing effect and pharmacology. Exp Eye Res. 1992; 54: 987-998.
- Rieck P, Assouline M, Hartmann C, Pouliquen Y, Courtois Y. [Effect of recombinant human basic fibroblast growth factor (rh-bFGF) on wound healing of the corneal epithelium]. Ophthalmologe. 1993; 90: 646-651.
- Carrington LM, Boulton M. Hepatocyte growth factor and keratinocyte growth factor regulation of epithelial and stromal corneal wound healing. J Cataract Refract Surg. 2005; 31: 412-423.
- Jester JV, Barry-Lane PA, Petroll WM, Olsen DR, Cavanagh HD. Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF beta in the rabbit. Cornea. 1997; 16: 177-187.
- Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000; 257: 180-189.
- `Nakamura M, Ofuji K, Chikama T, Nishida T. Combined effects of substance P and insulin-like growth factor-1 on corneal epithelial wound closure of rabbit in vivo. Curr Eye Res. 1997; 16: 275-278.
- Chikama T, Fukuda K, Morishige N, Nishida T. Treatment of neurotrophic keratopathy with substance-P-derived peptide (FGLM) and insulin-like growth factor I. Lancet. 1998; 351: 1783-1784.
- Li X, Li Z, Qiu L, Zhao C, Hu Z. Nerve growth factor modulate proliferation of cultured rabbit corneal endothelial cells and epithelial cells. J Huazhong Univ Sci Technolog Med Sci. 2005; 25: 575-577.
- Aloe L, Tirassa P, Lambiase A. The topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcers. Pharmacol Res. 2008; 57: 253-258.
- Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998; 338: 1174-1180.
- Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000; 107: 1347-1351; discussion 1351-1342.
- Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol. 2002; 50: 393-413.
- Xu KP, Yin J, Yu FS. Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor. Invest Ophthalmol Vis Sci. 2007; 48: 636-643.
- Hoppenreijs VP, Pels E, Vrensen GF, Treffers WF. Effects of platelet-derived growth factor on endothelial wound healing of human corneas. Invest Ophthalmol Vis Sci. 1994; 35: 150-161.
- Haber M, Cao Z, Panjwani N, Bedenice D, Li WW et al. Effects of growth factors (EGF, PDGF-BB and TGF-beta 1) on cultured equine epithelial cells and keratocytes: implications for wound healing. Vet Ophthalmol. 2003; 6: 211-217.
- Stramer BM, Fini ME. Uncoupling keratocyte loss of corneal crystallin from markers of fibrotic repair. Invest Ophthalmol Vis Sci. 2004; 45: 4010-4015.
- Davies BW, Panday V, Caldwell M, Scribbick F, Reilly CD. Effect of topical immunomodulatory interleukin 1 receptor antagonist therapy on corneal healing in New Zealand white rabbits (Oryctolagus cunniculus) after photorefractive keratectomy. Arch Ophthalmol. 2011; 129: 909-913.
- Garrana RM, Zieske JD, Assouline M, Gipson IK. Matrix metalloproteinases in epithelia from human recurrent corneal erosion. Invest Ophthalmol Vis Sci. 1999; 40: 1266-1270.
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006; 441: 235-238.
- Wang X, Kamiyama K, Iguchi I, Kita M, Imanishi J. Enhancement of fibronectin-induced migration of corneal epithelial cells by cytokines. Invest Ophthalmol Vis Sci. 1994; 35: 4001-4007.
- Kimura K. [Molecular mechanism of the disruption of barrier function in cultured human corneal epithelial cells induced by tumor necrosis factor-alpha, a proinflammatory cytokine]. Nihon Ganka Gakkai Zasshi. 2010; 114: 935-943.
- Jumblatt MM, Neufeld AH. A tissue culture assay of corneal epithelial wound closure. Invest Ophthalmol Vis Sci. 1986; 27: 8-13.
- Sakemi F, Nakayasu K, Okisaka S. [Mitotic activity of rabbit corneal epithelium in vitro]. Nihon Ganka Gakkai Zasshi. 1991; 95: 721-727.
- Jumblatt MM, Neufeld AH. Characterization of cyclic AMP-mediated wound closure of the rabbit corneal epithelium. Curr Eye Res. 1981; 1: 189-195.
- Ghoghawala SY, Mannis MJ, Pullar CE, Rosenblatt MI, Isseroff RR. Beta2-adrenergic receptor signaling mediates corneal epithelial wound repair. Invest Ophthalmol Vis Sci. 2008; 49: 1857-1863.
- Wang XL, Elgjo K, Haaskjold E. Regeneration of rat corneal epithelium is delayed by the inhibitory epidermal pentapeptide (EPP). Acta Ophthalmol Scand. 1996; 74: 361-363.
- Elgjo K, Reichelt KL. Beta-receptor blockade by propranolol modifies the effect of the inhibitory, endogenous epidermal pentapeptide on epidermal cell flux at the G2-M transition but not at the G1-S transition. Epithelial Cell Biol. 1994; 3: 32-37.
- Haruta Y, Ohashi Y, Matsuda S. Corneal epithelial deficiency induced by the use of beta-blocker eye drops. Eur J Ophthalmol. 1997; 7: 334-339.
- Murphy CJ, Campbell S, Araki-Sasaki K, Marfurt CF. Effect of norepinephrine on proliferation, migration, and adhesion of SV-40 transformed human corneal epithelial cells. Cornea. 1998; 17: 529-536.
- Jones MA, Marfurt CF. Sympathetic stimulation of corneal epithelial proliferation in wounded and nonwounded rat eyes. Invest Ophthalmol Vis Sci. 1996; 37: 2535-2547.
- Krejci L, Harrison R. Epinephrine effects on corneal cells in tissue culture. Arch Ophthalmol. 1970; 83: 451-454.
- Perez E, Lopez-Briones LG, Gallar J, Belmonte C. Effects of chronic sympathetic stimulation on corneal wound healing. Invest Ophthalmol Vis Sci. 1987; 28: 221-224.
- Saika S, Miyamoto T, Yamanaka O, Kato T, Ohnishi Y et al. Therapeutic effect of topical administration of SN50, an inhibitor of nuclear factor-kappaB, in treatment of corneal alkali burns in mice. Am J Pathol. 2005; 166: 1393-1403.
- Nakamura M, Fujihara T, Mibu H, Hikida M. Arachidonic acid stimulates corneal epithelial migration. J Ocul Pharmacol. 1994; 10: 453-459.
- Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M et al. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005; 280: 15267-15278.
- Yamada M, Proia AD. 8(S)-hydroxyeicosatetraenoic acid is the lipoxygenase metabolite of arachidonic acid that regulates epithelial cell migration in the rat cornea. Cornea. 2000; 19: S13-20.
- Edelhauser HF, Geroski DH, Woods WD, Holley GP, Laniado-Schwartzman M. Swelling in the isolated perfused cornea induced by 12(R)hydroxyeicosatetraenoic acid. Invest Ophthalmol Vis Sci. 1993; 34: 2953-2961.
- Sotozono C, He J, Tei M, Honma Y, Kinoshita S. Effect of metalloproteinase inhibitor on corneal cytokine expression after alkali injury. Invest Ophthalmol Vis Sci. 1999; 40: 2430-2434.
- Paterson CA, Wells JG, Koklitis PA, Higgs GA, Docherty AJ. Recombinant tissue inhibitor of metalloproteinases type 1 suppresses alkali-burn-induced corneal ulceration in rabbits. Invest Ophthalmol Vis Sci. 1994; 35: 677-684.
- Watanabe M, Kondo S, Mizuno K, Yano W, Nakao H et al. Promotion of corneal epithelial wound healing in vitro and in vivo by annexin A5. Invest Ophthalmol Vis Sci. 2006; 47: 1862-1868.
- Wang Z, Sosne G, Kurpakus-Wheater M. Plasminogen activator inhibitor-1 (PAI-1) stimulates human corneal epithelial cell adhesion and migration in vitro. Exp Eye Res. 2005; 80: 1-8.
- Williams PB, Crouch ER, Jr., Crouch ER, Mazaheri M. Topical aminocaproic acid facilitates reepithelialization of persistent epithelial defects. Curr Eye Res. 1999; 18: 150-157.
- Dougherty JM, Mcculley JP, Silvany RE, Meyer DR. The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Invest Ophthalmol Vis Sci. 1991; 32: 2970-2975.
- Smith VA, Cook SD. Doxycycline-a role in ocular surface repair. Br J Ophthalmol. 2004; 88: 619-625.
- Hope-Ross MW, Chell PB, Kervick GN, Mcdonnell PJ, Jones HS. Oral tetracycline in the treatment of recurrent corneal erosions. Eye (Lond). 1994; 8 ( Pt 4): 384-388.
- Perry HD, Hodes LW, Seedor JA, Donnenfeld ED, Mcnamara TF et al. Effect of doxycycline hyclate on corneal epithelial wound healing in the rabbit alkali-burn model. Preliminary observations. Cornea. 1993; 12: 379-382.
- Xiao O, Xie ZL, Lin BW, Yin XF, Pi RB et al. Minocycline inhibits alkali burn-induced corneal neovascularization in mice. PLoS One. 2012; 7: e41858.
- Lebrun-Vignes B, Kreft-Jais C, Castot A, Chosidow O, French Network of Regional Centers Of P. Comparative analysis of adverse drug reactions to tetracyclines: results of a French national survey and review of the literature. Br J Dermatol. 2012; 166: 1333-1341.
- Hara Y, Matsuura T, Tsukamoto M, Ishizaka S, Saishin M. Effect of tetra-peptide isolated from interleukin 1 (IL-1) on corneal epithelial wound healing in the rabbit. Exp Eye Res. 2001; 72: 107-113.
- Yamada N, Matsuda R, Morishige N, Yanai R, Chikama TI et al. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol. 2008; 92: 896-900.
- Huang LC, Petkova TD, Reins RY, Proske RJ, Mcdermott AM. Multifunctional roles of human cathelicidin (LL-37) at the ocular surface. Invest Ophthalmol Vis Sci. 2006; 47: 2369-2380.
- Yin J, Yu FS. LL-37 via EGFR transactivation to promote high glucose-attenuated epithelial wound healing in organ-cultured corneas. Invest Ophthalmol Vis Sci. 2010; 51: 1891-1897.
- Koczulla R, Von Degenfeld G, Kupatt C, Krotz F, Zahler S et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003; 111: 1665-1672.
- De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000; 192: 1069-1074.
- Pereira HA, Ruan X, Gonzalez ML, Tsyshevskaya-Hoover I, Chodosh J. Modulation of corneal epithelial cell functions by the neutrophil-derived inflammatory mediator CAP37. Invest Ophthalmol Vis Sci. 2004; 45: 4284-4292.
- Pereira HA, Shafer WM, Pohl J, Martin LE, Spitznagel JK. CAP37, a human neutrophil-derived chemotactic factor with monocyte specific activity. J Clin Invest. 1990; 85: 1468-1476.
- Bonfiglio V, Camillieri G, Avitabile T, Leggio GM, Drago F. Effects of the COOH-terminal tripeptide alpha-MSH(11-13) on corneal epithelial wound healing: role of nitric oxide. Exp Eye Res. 2006; 83: 1366-1372.
- Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994; 94: 2036-2044.
- Saika S, Uenoyama K, Hiroi K, Ooshima A. L-ascorbic acid 2-phosphate enhances the production of type I and type III collagen peptides in cultured rabbit keratocytes. Ophthalmic Res. 1992; 24: 68-72.
- Levinson RA, Paterson CA, Pfister RR. Ascorbic acid prevents corneal ulceration and perforation following experimental alkali burns. Invest Ophthalmol. 1976; 15: 986-993.
- Haddox JL, Pfister RR, Slaughter SE. An excess of topical calcium and magnesium reverses the therapeutic effect of citrate on the development of corneal ulcers after alkali injury. Cornea. 1996; 15: 191-195.
- Pfister RR, Nicolaro ML, Paterson CA. Sodium citrate reduces the incidence of corneal ulcerations and perforations in extreme alkali-burned eyes--acetylcysteine and ascorbate have no favorable effect. Invest Ophthalmol Vis Sci. 1981; 21: 486-490.
- Urgancioglu B, Bilgihan K, Engin D, Cirak MY, Hondur A et al. Topical N-acetylcysteine reduces interleukin-1-alpha in tear fluid after laser subepithelial keratectomy. Eur J Ophthalmol. 2009; 19: 554-559.
- Aldavood SJ, Behyar R, Sarchahi AA, Rad MA, Noroozian I et al. Effect of acetylcysteine on experimental corneal wounds in dogs. Ophthalmic Res. 2003; 35: 319-323.
- Sarchahi AA, Maimandi A, Tafti AK, Amani M. Effects of acetylcysteine and dexamethasone on experimental corneal wounds in rabbits. Ophthalmic Res. 2008; 40: 41-48.
- Ainscough SL, Barnard Z, Upton Z, Harkin DG. Vitronectin supports migratory responses of corneal epithelial cells to substrate bound IGF-I and HGF, and facilitates serum-free cultivation. Exp Eye Res. 2006; 83: 1505-1514.
- Bashkaran K, Zunaina E, Bakiah S, Sulaiman SA, Sirajudeen K et al. Anti-inflammatory and antioxidant effects of Tualang honey in alkali injury on the eyes of rabbits: experimental animal study. BMC Complement Altern Med. 2011; 11: 90.
- Callizo J, Cervello I, Mayayo E, Mallol J. Inefficacy of collagen shields in the rabbit corneal wound-healing process. Cornea. 1996; 15: 258-262.
- Celik T, Katircioglu YA, Singar E, Kosker M, Budak K et al. Clinical outcomes of amniotic membrane transplantation in patients with corneal and conjunctival disorders. Semin Ophthalmol. 2013; 28: 41-45.
- Chung JH, Kang YG, Kim HJ. Effect of 0.1% dexamethasone on epithelial healing in experimental corneal alkali wounds: morphological changes during the repair process. Graefes Arch Clin Exp Ophthalmol. 1998; 236: 537-545.
- Coday MP, Warner MA, Jahrling KV, Rubin PA. Acquired monocular vision: functional consequences from the patient's perspective. Ophthal Plast Reconstr Surg. 2002; 18: 56-63.
- Den S, Sotozono C, Kinoshita S, Ikeda T. Efficacy of early systemic betamethasone or cyclosporin A after corneal alkali injury via inflammatory cytokine reduction. Acta Ophthalmol Scand. 2004; 82: 195-199.
- Dignass A, Lynch-Devaney K, Kindon H, Thim L, Podolsky DK. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994; 94: 376-383.
- Donshik PC, Berman MB, Dohlman CH, Gage J, Rose J. Effect of topical corticosteroids on ulceration in alkali-burned corneas. Arch Ophthalmol. 1978; 96: 2117-2120.
- Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980; 286: 264-265.
- Garrett Q, Simmons PA, Xu S, Vehige J, Zhao Z et al. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2007; 48: 1559-1567.
- Garrett Q, Xu S, Simmons PA, Vehige J, Xie RZ et al. Carboxymethyl cellulose stimulates rabbit corneal epithelial wound healing. Curr Eye Res. 2008; 33: 567-573.
- Goke MN, Cook JR, Kunert KS, Fini ME, Gipson IK et al. Trefoil peptides promote restitution of wounded corneal epithelial cells. Exp Cell Res. 2001; 264: 337-344.
- Gomes JA, Amankwah R, Powell-Richards A, Dua HS. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br J Ophthalmol. 2004; 88: 821-825.
- Gupta AG, Hirakata A, Proia AD. Effect of inhibitors of arachidonic acid metabolism on corneal reepithelialization in the rat. Exp Eye Res. 1993; 56: 701-708.
- Hallberg CK, Trocme SD, Ansari NH. Acceleration of corneal wound healing in diabetic rats by the antioxidant trolox. Res Commun Mol Pathol Pharmacol. 1996; 93: 3-12.
- Hayashi Y, Call MK, Chikama T, Liu H, Carlson EC et al. Lumican is required for neutrophil extravasation following corneal injury and wound healing. J Cell Sci. 2010; 123: 2987-2995.
- Kampfer C, Saller S, Windschuttl S, Berg D, Berg U et al. Pigment-Epithelium Derived Factor (PEDF) and the human ovary: A role in the generation of ROS in granulosa cells. Life Sci. 2013.
- Kim YH, Jin KH, Choi S, Park HK. Molecular and chemical investigations and comparisons of biomaterials for ocular surface regeneration. Microsc Res Tech. 2013.
- Konomi K, Satake Y, Shimmura S, Tsubota K, Shimazaki J. Long-term results of amniotic membrane transplantation for partial limbal deficiency. Cornea. 2013; 32: 1110-1115.
- Lawlor M, Dobbins T, Thomas KA, Billson F. Consent for corneal donation: the effect of age of the deceased, registered intent and which family member is asked about donation. Br J Ophthalmol. 2006; 90: 1383-1385.
- Lowenstein EB, Lowenstein EJ. Isotretinoin systemic therapy and the shadow cast upon dermatology's downtrodden hero. Clin Dermatol. 2011; 29: 652-661.
- Luo H, Lu Y, Wu T, Zhang M, Zhang Y et al. Construction of tissue-engineered cornea composed of amniotic epithelial cells and acellular porcine cornea for treating corneal alkali burn. Biomaterials. 2013; 34: 6748-6759.
- Miyanaga M, Miyai T, Nejima R, Maruyama Y, Miyata K et al. Effect of bromfenac ophthalmic solution on ocular inflammation following cataract surgery. Acta Ophthalmol. 2009; 87: 300-305.
- Nakamura M, Nishida T. Synergistic effects of hyaluronan and fibronectin on epithelial migration in rabbit cornea in vitro. Cornea. 1999; 18: 686-692.
- Nakamura M, Sato N, Chikama TI, Hasegawa Y, Nishida T. Hyaluronan facilitates corneal epithelial wound healing in diabetic rats. Exp Eye Res. 1997; 64: 1043-1050.
- Nishida T, Ohashi Y, Awata T, Manabe R. Fibronectin. A new therapy for corneal trophic ulcer. Arch Ophthalmol. 1983; 101: 1046-1048.
- Parmar DN, Alizadeh H, Awwad S, Bowman RW, Cavanagh HD et al. Contact lens-based expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction: crossover control. Transplantation. 2010; 89: 483; author reply 484.
- Pattamatta U, Willcox M, Stapleton F, Cole N, Garrett Q. Bovine Lactoferrin Stimulates Human Corneal Epithelial Alkali Wound Healing in Vitro. Invest Ophthalmol Vis Sci. 2008; 5: 5.
- Paulsen FP, Woon CW, Varoga D, Jansen A, Garreis F et al. Intestinal trefoil factor/TFF3 promotes re-epithelialization of corneal wounds. J Biol Chem. 2008; 283: 13418-13427.
- Qiu P, Kurpakus-Wheater M, Sosne G. Matrix metalloproteinase activity is necessary for thymosin beta 4 promotion of epithelial cell migration. J Cell Physiol. 2007; 212: 165-173./a>
- Robin JB, Keys CL, Kaminski LA, Viana MA. The effect of collagen shields on rabbit corneal reepithelialization after chemical debridement. Invest Ophthalmol Vis Sci. 1990; 31: 1294-1300.
- Rodrigues S, Van Aken E, Van Bocxlaer S, Attoub S, Nguyen QD et al. Trefoil peptides as proangiogenic factors in vivo and in vitro: implication of cyclooxygenase-2 and EGF receptor signaling. Faseb J. 2003; 17: 7-16.
- Saika S, Shiraishi A, Liu CY, Funderburgh JL, Kao CW et al. Role of lumican in the corneal epithelium during wound healing. J Biol Chem. 2000; 275: 2607-2612.
- Saud EE, Moraes HV, Jr., Marculino LG, Gomes JA, Allodi S et al. Clinical and histopathological outcomes of subconjunctival triamcinolone injection for the treatment of acute ocular alkali burn in rabbits. Cornea. 2012; 31: 181-187.
- Seomun Y, Joo CK. Lumican induces human corneal epithelial cell migration and integrin expression via ERK 1/2 signaling. Biochem Biophys Res Commun. 2008; 372: 221-225.
- Soong HK, Martin NF, Wagoner MD, Alfonso E, Mandelbaum SH et al. Topical retinoid therapy for squamous metaplasia of various ocular surface disorders. A multicenter, placebo-controlled double-masked study. Ophthalmology. 1988; 95: 1442-1446.
- Sosne G, Qiu P, Kurpakus-Wheater M. Thymosin beta-4 and the eye: I can see clearly now the pain is gone. Ann N Y Acad Sci. 2007; 1112: 114-122.
- Srinivasan BD, Kulkarni PS, Bhat SP. Differential protein synthesis in steroid-treated ocular surface epithelium. Invest Ophthalmol Vis Sci. 1986; 27: 1005-1009.
- Stamler JF, Tse DT. A simple and reliable technique for permanent lateral tarsorrhaphy. Arch Ophthalmol. 1990; 108: 125-127.
- Tani E, Katakami C, Negi A. Effects of various eye drops on corneal wound healing after superficial keratectomy in rabbits. Jpn J Ophthalmol. 2002; 46: 488-495.
- Tjebbes GW, Van Delft JL, Van Haeringen NJ. Production of lipid mediators in experimental keratitis of rabbit eye. J Lipid Mediat. 1993; 8: 87-93.
- Tseng SC, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998; 116: 431-441.
- Uno K, Hayashi H, Kuroki M, Uchida H, Yamauchi Y et al. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem Biophys Res Commun. 2004; 315: 928-934.
- Vetrugno M, Maino A, Cardia G, Quaranta GM, Cardia L. A randomised, double masked, clinical trial of high dose vitamin A and vitamin E supplementation after photorefractive keratectomy. Br J Ophthalmol. 2001; 85: 537-539.
- Williams KA, Roder D, Esterman A, Muehlberg SM, Coster DJ. Factors predictive of corneal graft survival. Report from the Australian Corneal Graft Registry. Ophthalmology. 1992; 99: 403-414.
- Willoughby CE, Batterbury M, Kaye SB. Collagen corneal shields. Surv Ophthalmol. 2002; 47: 174-182.
- Yang G, Espandar L, Mamalis N, Prestwich GD. A cross-linked hyaluronan gel accelerates healing of corneal epithelial abrasion and alkali burn injuries in rabbits. Vet Ophthalmol. 2010; 13: 144-150.
- Yeh LK, Chen WL, Li W, Espana EM, Ouyang J et al. Soluble lumican glycoprotein purified from human amniotic membrane promotes corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2005; 46: 479-486.
- Pattamatta U, Willcox M, Stapleton F, Garrett Q. Bovine lactoferrin promotes corneal wound healing and suppresses IL-1 expression in alkali wounded mouse cornea. Curr Eye Res. 2013; 38: 1110-1117.
- Cameron JD, Hagen ST, Waterfield RR, Furcht LT. Effects of matrix proteins on rabbit corneal epithelial cell adhesion and migration. Curr Eye Res. 1988; 7: 293-301.
- Cao Z, Said N, Wu HK, Kuwabara I, Liu FT et al. Galectin-7 as a potential mediator of corneal epithelial cell migration. Arch Ophthalmol. 2003; 121: 82-86.
- Chung JH, Kim WK, Lee JS, Pae YS, Kim HJ. Effect of topical Na-hyaluronan on hemidesmosome formation in n-heptanol-induced corneal injury. Ophthalmic Res. 1998; 30: 96-100.
- Donnenfeld ED, Selkin BA, Perry HD, Moadel K, Selkin GT et al. Controlled evaluation of a bandage contact lens and a topical nonsteroidal anti-inflammatory drug in treating traumatic corneal abrasions. Ophthalmology. 1995; 102: 979-984.
- Frantz JM, Dupuy BM, Kaufman HE, Beuerman RW. The effect of collagen shields on epithelial wound healing in rabbits. Am J Ophthalmol. 1989; 108: 524-528.
- Fraunfelder FW, Cabezas M. Treatment of recurrent corneal erosion by extended-wear bandage contact lens. Cornea. 2011; 30: 164-166.
- Gordon JF, Johnson P, Musch DC. Topical fibronectin ophthalmic solution in the treatment of persistent defects of the corneal epithelium. Chiron Vision Fibronectin Study Group. Am J Ophthalmol. 1995; 119: 281-287.
- Ho TC, Chen SL, Wu JY, Ho MY, Chen LJ et al. PEDF promotes self-renewal of limbal stem cell and accelerates corneal epithelial wound healing. Stem Cells. 2013; 31: 1775-1784.
- Khokhar S, Natung T, Sony P, Sharma N, Agarwal N et al. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea. 2005; 24: 654-660.
- Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000; 20: 173-177.
- Mcculley JP, Horowitz B, Husseini ZM, Horowitz M. Topical fibronectin therapy of persistent corneal epithelial defects. Fibronectin Study Group. Trans Am Ophthalmol Soc. 1993; 91: 367-386; discussion 386-390.
- Mitchell J: Ocular emergencies. In: Emergency Medicine: A Comprehensive Study Guide. 5th edn. Edited by Tintinalli J, Kelen G, Stapczynsk iJ. New York: McGraw-Hill; 2000: 1501-1518.
- Naik MN, Gangopadhyay N, Fernandes M, Murthy R, Honavar SG. Anterior chemodenervation of levator palpebrae superioris with botulinum toxin type-A (Botox) to induce temporary ptosis for corneal protection. Eye (Lond). 2008; 22: 1132-1136.
- Nishida T, Nakagawa S, Awata T, Tani Y, Manabe R. Fibronectin eyedrops for traumatic recurrent corneal lesion. Lancet. 1983; 2: 521-522.
- Patel GM, Chuang AZ, Kiang E, Ramesh N, Mitra S et al. Epithelial healing rates with topical ciprofloxacin, ofloxacin, and ofloxacin with artificial tears after photorefractive keratectomy. J Cataract Refract Surg. 2000; 26: 690-694.
- Shimazaki J, Yang HY, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997; 104: 2068-2076.
- Srinivasan BD, Kulkarni PS. The role of arachidonic acid metabolites in the mediation of the polymorphonuclear leukocyte response following corneal injury. Invest Ophthalmol Vis Sci. 1980; 19: 1087-1093.
- Tandon R, Gupta N, Kalaivani M, Sharma N, Titiyal JS et al. Amniotic membrane transplantation as an adjunct to medical therapy in acute ocular burns. Br J Ophthalmol. 2011; 95: 199-204.
- Teenan DW, Sim KT, Hawksworth NR. Outcomes of corneal transplantation: a corneal surgeon vs the general ophthalmologist. Eye (Lond). 2003; 17: 727-730.
- Turner A, Rabiu M. Patching for corneal abrasion. Cochrane Database Syst Rev2006: CD004764.
- Vetrugno M, Maino A, Quaranta GM, Cardia L. The effect of early steroid treatment after PRK on clinical and refractive outcomes. Acta Ophthalmol Scand. 2001; 79: 23-27.
- Wagoner MD, Steinert RF. Temporary tarsorrhaphy enhances reepithelialization after epikeratoplasty. Arch Ophthalmol. 1988; 106: 13-14.
- Watson SL, Lee MH, Barker NH. Interventions for recurrent corneal erosions. Cochrane Database Syst Rev. 2012; 9: CD001861.
- Williams R, Buckley RJ. Pathogenesis and treatment of recurrent erosion. Br J Ophthalmol. 1985; 69: 435-437.
- Wong VW, Chi SC, Lam DS. Diamond burr polishing for recurrent corneal erosions: results from a prospective randomized controlled trial. Cornea. 2009; 28: 152-156.
- Yulek F, Ozdek S, Gurelik G, Hasanreisoglu B. Effect of topical steroids on corneal epithelial healing after vitreoretinal surgery. Acta Ophthalmol Scand. 2006; 84: 319-322.
- Cho YK, Huang W, Kim GY, Lim BS. Comparison of autologous serum eye drops with different diluents. Curr Eye Res. 2013; 38: 9-17.
- Yamada N, Yanai R, Nakamura M, Inui M, Nishida T. Role of the C domain of IGFs in synergistic promotion, with a substance P-derived peptide, of rabbit corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2004; 45: 1125-1131.
- Saika S, Uenoyama K, Hiroi K, Tanioka H, Takase K et al. Ascorbic acid phosphate ester and wound healing in rabbit corneal alkali burns: epithelial basement membrane and stroma. Graefes Arch Clin Exp Ophthalmol. 1993; 231: 221-227.
- Kruse FE, Tseng SC. Retinoic acid regulates clonal growth and differentiation of cultured limbal and peripheral corneal epithelium. Invest Ophthalmol Vis Sci. 1994; 35: 2405-2420.
- Nishida T, Nakagawa S, Manabe R. Clinical evaluation of fibronectin eyedrops on epithelial disorders after herpetic keratitis. Ophthalmology. 1985; 92: 213-216.
- Kabata T, Ishibashi Y, Honmura S, Akama T, Yatohgo T et al. [Effect of vitronectin on the healing of rabbit corneal epithelial damage]. Nihon Ganka Gakkai Zasshi. 1990; 94: 457-461.
- Algawi K, Agrell B, Goggin M, O'keefe M. Randomized clinical trial of topical sodium hyaluronate after excimer laser photorefractive keratectomy. J Refract Surg. 1995; 11: 42-44.
- Chahud F, Ramalho LN, Ramalho FS, Haddad A, Roque-Barreira MC. The lectin KM+ induces corneal epithelial wound healing in rabbits. Int J Exp Pathol. 2009; 90: 166-173.
- Cao Z, Said N, Amin S, Wu HK, Bruce A et al. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem. 2002; 277: 42299-42305.
- Gipson IK, Anderson RA. Effect of lectins on migration of the corneal epithelium. Invest Ophthalmol Vis Sci. 1980; 19: 341-349.
- Dunn SP, Heidemann DG, Chow CY, Crockford D, Turjman N et al. Treatment of chronic nonhealing neurotrophic corneal epithelial defects with thymosin beta 4. Arch Ophthalmol. 2010; 128: 636-638.
- Dunn SP, Heidemann DG, Chow CY, Crockford D, Turjman N et al. Treatment of chronic nonhealing neurotrophic corneal epithelial defects with thymosin beta4. Ann N Y Acad Sci. 2010; 1194: 199-206.
- Weaver CS, Terrell KM. Evidence-based emergency medicine. Update: do ophthalmic nonsteroidal anti-inflammatory drugs reduce the pain associated with simple corneal abrasion without delaying healing? Ann Emerg Med. 2003; 41: 134-140.
- Badala F, Fioretto M, Macri A. Effect of topical 0.1% indomethacin solution versus 0.1% fluorometholon acetate on ocular surface and pain control following laser subepithelial keratomileusis (LASEK). Cornea. 2004; 23: 550-553.
- Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Lalitha P et al. Corticosteroids for Bacterial Keratitis: The Steroids for Corneal Ulcers Trial (SCUT). Arch Ophthalmol. 2011.
- Uwaydat S, Jha P, Tytarenko R, Brown H, Wiggins M et al. The use of topical honey in the treatment of corneal abrasions and endotoxin-induced keratitis in an animal model. Curr Eye Res. 2011; 36: 787-796.
- Engle AT, Laurent JM, Schallhorn SC, Toman SD, Newacheck JS et al. Masked comparison of silicone hydrogel lotrafilcon A and etafilcon A extended-wear bandage contact lenses after photorefractive keratectomy. J Cataract Refract Surg. 2005; 31: 681-686.
- Lim P, Ridges R, Jacobs DS, Rosenthal P. Treatment of persistent corneal epithelial defect with overnight wear of a prosthetic device for the ocular surface. Am J Ophthalmol. 2013; 156: 1095-1101.
- Ruffini JJ, Aquavella JV, Locascio JA. Effect of collagen shields on corneal epithelialization following penetrating keratoplasty. Ophthalmic Surg. 1989; 20: 21-25.
- Di Girolamo N, Bosch M, Zamora K, Coroneo MT, Wakefield D et al. A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation. 2009; 87: 1571-1578.
- Wilson SE, Esposito A. Focus on molecules: interleukin-1: a master regulator of the corneal response to injury. Exp Eye Res. 2009; 89: 124-125.
- Nishida T, Nakamura M, Mishima H, Otori T, Hikida M. Interleukin 6 facilitates corneal epithelial wound closure in vivo. Arch Ophthalmol1992; 110: 1292-1294.
- Fini ME, Cook JR, Mohan R. Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res. 1998; 290: S12-23.
- Kao WW, Kao CW, Kaufman AH, Kombrinck KW, Converse RL et al. Healing of corneal epithelial defects in plasminogen- and fibrinogen-deficient mice. Invest Ophthalmol Vis Sci. 1998; 39: 502-508.
- Watanabe M, Yano W, Kondo S, Hattori Y, Yamada N et al. Up-regulation of urokinase-type plasminogen activator in corneal epithelial cells induced by wounding. Invest Ophthalmol Vis Sci. 2003; 44: 3332-3338.
- Hayashi K, Berman M, Smith D, El-Ghatit A, Pease S et al. Pathogenesis of corneal epithelial defects: role of plasminogen activator. Curr Eye Res. 1991; 10: 381-398.
- Fini ME, Parks WC, Rinehart WB, Girard MT, Matsubara M et al. Role of matrix metalloproteinases in failure to re-epithelialize after corneal injury. Am J Pathol. 1996; 149: 1287-1302.
- Hossain P. The corneal melting point. Eye (Lond). 2012; 26: 1029-1030.
- Yamada N, Yanai R, Kawamoto K, Nagano T, Nakamura M et al. Promotion of corneal epithelial wound healing by a tetrapeptide (SSSR) derived from IGF-1. Invest Ophthalmol Vis Sci. 2006; 47: 3286-3292.
- Appling WD, O'brien WR, Johnston DA, Duvic M. Synergistic enhancement of type I and III collagen production in cultured fibroblasts by transforming growth factor-beta and ascorbate. FEBS Lett. 1989; 250: 541-544.
- Gipson IK, Kiorpes TC, Brennan SJ. Epithelial sheet movement: effects of tunicamycin on migration and glycoprotein synthesis. Dev Biol. 1984; 101: 212-220.
- Amico C, Yakimov M, Catania MV, Giuffrida R, Pistone M et al. Differential expression of cyclooxygenase-1 and cyclooxygenase-2 in the cornea during wound healing. Tissue Cell. 2004; 36: 1-12.
- Kossendrup D, Wiederholt M, Hoffmann F. Influence of cyclosporin A, dexamethasone, and benzalkonium chloride (BAK) on corneal epithelial wound healing in the rabbit and guinea pig eye. Cornea. 1985; 4: 177-181.
- Machat JJ. Double-blind corticosteroid trial in identical twins following photorefractive keratectomy. Refract Corneal Surg. 1993; 9: S105-107.