Research Article
Austin J Clin Ophthalmol. 2014;1(4): 1021.
Efficacy of Using the Icare® Rebound Tonometer Combined with a Simple Supplementary Device for the Self-Examination of Intraocular Pressure
Ayako Iwama, Kazuhiko Mori*, Shigeta Naruse, Yoko Ikeda and Shigeru Kinoshita
Department of Ophthalmology, Kyoto Prefectural University of Medicine, Japan
*Corresponding author: Kazuhiko Mori, Department of Ophthalmology, Kyoto Prefectural University of Medicine, 465 Kajii-cho, Hirokoji-agaru, Kawaramachidori, Kamigyo-ku, Kyoto 602-0841, Japan
Received: April 10, 2014; Accepted: May 10, 2014; Published: May 13, 2014
Abstract
Purpose : To evaluate the efficacy of using the Icare® (Icare Finland Oy, Vantaa, Finland) rebound tonometer combined with a simple supplementary device for self–measurement of intraocular pressure (IOP).
Subjects and Methods : This study involved 104 eyes of 52, healthy righthanded volunteers. IOP was consecutively measured using the Icare® by an ophthalmologist and by the participants themselves through self–examination. To accurately position the Icare® probe against the center of the cornea during self–examination, a colored board with a 7–mm–diameter central hole affixed to an eyeglass frame was used. Participants were then instructed to wear the frame, insert the Icare® probe into the hole, and self–measure the IOP of both eyes.
Results : Correlation was found between the IOP readings obtained by the ophthalmologist and those obtained by self–examination. The correlation was stronger when the position between the probe and cornea was in better alignment. In 42 eyes, the probe was precisely directed at the center of the cornea, and those readings were found to be strongly correlated.
Conclusions : The Icare® combined with a simple supplementary device proved effective for producing reliable IOP readings, thus providing the possibility of using the Icare® for self–examination of IOP in both the home and clinical settings.
Keywords : Intraocular pressure; Rebound tonometer; Self examination
Introduction
It is well known that glaucoma is a leading cause of permanent blindness worldwide [1]. Although Intraocular Pressure (IOP) is no longer considered to be a cause of glaucoma, it is still an important risk factor for its development [2,3]. In addition, IOP measurement is an important indicator by which to evaluate the efficacy of various treatment methods [4]. Therefore, tonometry is still considered to be one of the gold–standard procedures associated with the treatment of glaucoma in the clinical setting. Despite the availability of numerous portable devices, the majority of ophthalmologists rely solely on Goldmann Applanation Tonometry (GAT) as the standard method for measuring IOP, a method in which the obtained IOP readings are used to guide decisions regarding patient management [6]. However, it has been reported that IOP can vary throughout the day and that IOP diurnal fluctuation could influence the diagnostic and prognostic evaluation of the glaucomatous disease [6]. Therefore, a portable and easy–to–use tonometer would be extremely valuable in the clinical setting, especially for the detection of diurnal changes in IOP, as well as for self–examination of IOP in the home [7,8].
Originally released in 2005, the Icare® (Icare Finland Oy, Vantaa, Finland) is a hand–held rebound tonometer that has a solenoid mechanism which launches a magnetized probe and detects its motion parameters during impact on the cornea [9,10]. This tonometer is light and compact, and can measure IOP without the need for topical anesthesia. Thus, the characteristics of this tonometer may prove to be ideal for the self–examination of IOP in the home setting. Rebound tonometry is based on a moving object impacting with the ocular surface while monitoring the motion parameters of that object [9,10]. When IOP is low, the deceleration of the probe is less than that observed when IOP is high. Consequently, the higher the IOP, the shorter the duration of the impact. IOP is calculated from the measurement of impact duration and maximum deceleration [9]. In order to obtain more accurate IOP readings with the Icare® (i.e., no error bars), extra care must be taken to ensure that the probe is held perpendicular to the center of the cornea and that the measurements are taken as quickly as possible, with minimal movement of the operator’s hand or the patient’s eye [11]. In addition, it is vital that the operator stand directly in front of the patient in order to ensure that the probe is not tilted off–axis in either the horizontal or vertical plane. Thus, this difficulty is one that needs to be resolved in order for the Icare® to be effectively and easily used as a home tonometer for the self–examination of IOP.
We previously examined whether there was any correlation between the Icare® tonometer IOP value when measured by an ophthalmologist and by the patients themselves through selfexamination. However, no strong correlation was found between the values measured by each method due to the difficulty that the patients experienced in accurately positioning the Icare® probe at the center of the cornea during self–examination (data not published).
In this present study, we evaluated the efficacy of using the Icare® tonometer combined with a simple supplementary device for the selfexamination of IOP. The latest Icare® model available for use in Japan was released in 2012, and it provides some advantages compared to the previous Icare® model. In specific, it offers the ability to check the IOP of patients while their spine is in a reclining position. The Icare® used in this present study has some merits compared to the latest type; i.e., it is lighter in weight, more easily to handle for selfexamination, and it is lower in price. Soon, a newer Icare® model that is specifically designed for self–examination will become available in Japan, however, the Icare® model used in this present study is stilluseful for the self examination of IOP by using a supplementary method.
Participants and Methods
In this present study, a total of 104 eyes of 52, healthy righthanded volunteers (45 males and 7 females; age range: 25–51 years, mean age: 36.0±7.7 years) were enrolled. Participants who regularly used soft contact lenses were instructed to not wear the lenses for at least 24 hours prior to participation in the study. All participants had no history of ocular surgery, were free of ocular symptoms, and were not taking any medication. Informed consent was obtained from all subjects after receiving a detailed explanation of the study protocol.
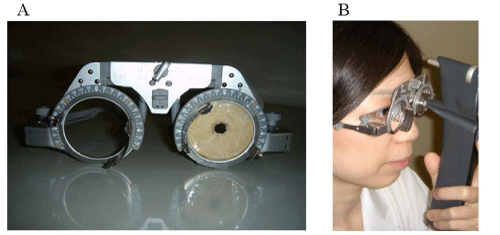
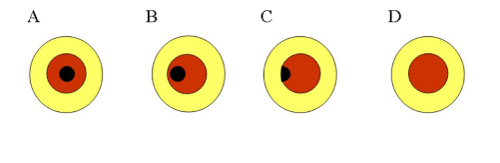
IOP was consecutively measured using the Icare® tonometer by an ophthalmologist (A.I.) and by the participants themselves. Prior to the examination, the participants were instructed in the proper use of the Icare®. In order to accurately position the Icare® probe against the center of the cornea during self–measurement, a single eyeglass frame which has a capability of changing the pupillary distance up to 56–70 mm was used. We adjust the glass in relation to the pupil distance for each patient prior to IOP measurement. A colored board with a 7–mm–diameter hole in its center was affixed to the eyeglass frame in place of the usual lens (Figure 1A). The participants were then instructed to wear the frame, insert the Icare® probe into the hole, and self–measure the IOP of both eyes with their most convenient method (Figure 1B). Patients always check their probe position by use of a mirror in order to insure that the measurements are taken correctly with the Icare. The accuracy of the self–measurements by the participants was categorized into four subgroups based upon whether the position of the hole was in alignment with the papillary center and whether the position of the probe–tip was in alignment with thecenter of the cornea (Figure 2). Consecutive measurements were then obtained from each eye until either a mean reading displaying a static P with no bar (P) was acquired, or a total of 6 readings were performed, regardless of standard deviation (SD). The total number of attempts needed to achieve a P reading was then recorded for each participant.
Figure 1 :(A) The single eyeglass frame used in this study. A colored board with a 7-mm-diameter hole in its center was affixed to the eyeglass frame in place of the usual clear lens. (B) Self-measurement of intraocular pressure (IOP) using the frame and Icare®. The participants were instructed to wear the frame, insert the Icare® probe into the hole, and then self-measure the IOP of both eyes using their most convenient method.
Figure 2:Schema of the four subgroups based upon whether the position of the hole was in alignment with the center of the pupil and whether the position of the probe-tip was in alignment with the center of the cornea. Yellow demonstrates the colored board. Brown demonstrates the iris which can be confirmed from the hole in the colored board. Black demonstrates the pupil which can be confirmed from the hole in the colored board. (A) The center of the hole and the center of the pupil are corresponding. (B) The position shifts from the center, though the whole area of the pupil can be confirmed from the hole. (C) Only a portion of the pupil can be confirmed from the hole. (D) The pupil cannot be confirmed from the hole, or the tip of the probe touches the eyelashes and the eyelid, or the distance between the tip of the probe and the center of the cornea is less than 3mm or more than 9mm.
After the participant’s self–measurement of IOP by Icare®, topical anesthesia (0.4% oxybuprocaine: Benoxil; Santen PharmaceuticalCo., Ltd., Osaka, Japan) was applied, and an ophthalmologist (A.I.)then measured the patient’s IOP with a Goldmann ApplanationTonometer (Haag–Streit AG, Konniz, Switzerland) calibrated prior to the inception of the study and placed over the center of the cornea. The IOP values were presented as mean ± SD, and a P–value less than 0.05 was considered statistically significant. IOP results were compared among those obtained by GAT, those obtained by Icare® as measured by the ophthalmologist, and those obtained by Icare® as measured by the participants. Post–hoc analysis was performed whenever a significant difference was found. The correlation between the IOP readings obtained by the ophthalmologist and those obtained by the participants during self–examination was evaluated using the Pearson correlation coefficients and regression analysis.
Results
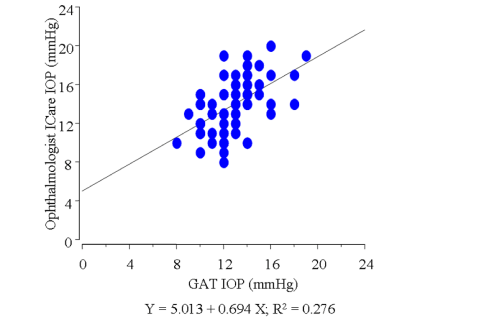
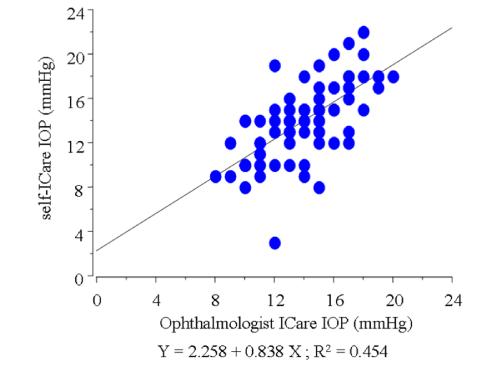
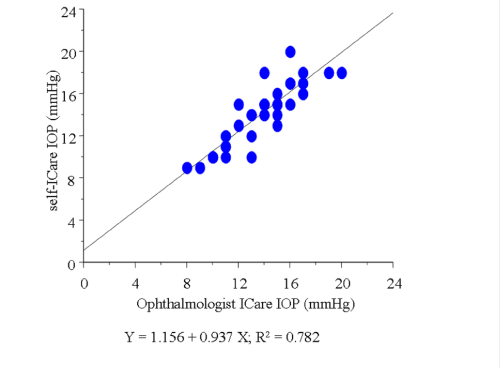
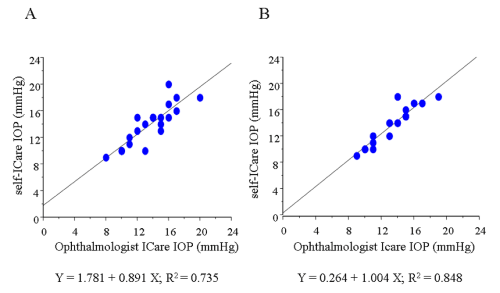
In this study, we excluded the data of 23 eyes in which the Icare® probe failed to touch the cornea due to the unsuitable distance or malposition of the probe (subgroup D, Figure 2). In the remaining 81 eyes, the average of IOP measured by GAT was 12.9±2.2 mmHg. The average of IOP using the Icare® tonometer was 14.0±2.7 mmHg by the ophthalmologist, and 13.7±3.7 mmHg by the participants themselves, respectively, which were higher than the average measured by GAT (P= 0.0046, 0.0131, respectively). In those 81 eyes, a slightly weak correlation was found between the IOP readings obtained by the ophthalmologist with GAT and those obtained by the ophthalmologist with Icare® (r = 0.525, Figure 3). In addition, a correlation was found between the IOP readings obtained by the ophthalmologist and those obtained by the participants during self–examination in those 81 eyes (r = 0.674, Figure 4). The correlation was stronger when the position between the probe and cornea was in better alignment. In 42 eyes,the probe touched the corneal center exactly, and those readings were strongly correlated (r = 0.884, Figure 5). In those 42 eyes, we also compared IOP readings between the right eyes and left eyes and found that the right eyes were in stronger correlation (r = 0.921 and 0.857, respectively; Figure 6).
Figure 3 : Graph demonstrating the correlation between the IOP readings obtained by the ophthalmologist with Goldman applanation tonometry and those obtained by the ophthalmologist using the Icare® in the 81 enrolled eyes.
Figure 4 : Graph demonstrating the correlation between the IOP readings obtained by the ophthalmologist and those obtained by the participants during self-examination using the Icare® in the 81 enrolled eyes.
Figure 5 : Graph demonstrating the strong correlation between the IOP readings obtained by the ophthalmologist and those obtained by the participants during self-examination in 42 eyes when the probe was precisely positioned toward the center of the cornea.
Figure 6 : Graphs demonstrating the correlation between the IOP readings obtained by the ophthalmologist and those obtained by the participants during self-examination with a comparison between the right eyes (n = 19) and left eyes (n = 23) in 42 eyes when the probe was precisely positioned toward the center of the cornea. (A) Left eyes. (B) Right eyes.
Discussion
The Icare® rebound tonometer, which uses the impact⁄induction principle, has recently become commercially available [9]. One characteristic of this tonometer is that the touch of the probe to the cornea is so quick that there is no need for any topical anesthesia. Moreover, the Icare® tonometer should prove useful for children, in whom contact tonometry has often been difficult and because some children are afraid of the air puff associated with noncontact tonometry. However, it should be noted that in order for the Icare® to be effectively and easily used as a home tonometer for the selfexamination of IOP, some difficulties associated with its usage still need to be resolved. In a recent study by Davies [12]. that evaluated the reliability and repeatability of IOP measurements using Icare®, they concluded that measurement of IOP in normal healthy subjects usingthe Icare® produced a small, statistically insignificant positive bias when compared with the GAT and that intersessional repeatability of IOP taken with the Icare® is poorer than that of IOP taken with the GAT. This poor repeatability is partly due to the fact that extra care must be taken to ensure that the probe is held perpendicular to the center of the cornea and that the measurements are taken as quickly as possible, with minimal movement of the operator or the patients’ eyes in order to obtain more accurate IOP readings with the Icare® [11]. That group also reported that it was necessary for the operatorto stand directly in front of the subject to ensure that the probe was not tilted off–axis either in the horizontal or vertical plane. In this study, we used a single eyeglass frame with an affixed colored boardwith a hole in its center in place of the usual clear lens as a new selfexamination method. By use of this method, the participants found it easy to insert the Icare® probe into the hole and to self–measure the IOP of both eyes using their most convenient method.
In a report by Abraham [13] the effectiveness of using the Icare® rebound tonometer was compared with that of GAT in the hands of experienced and inexperienced optometrists. In their report, two optometrists experienced with both the GAT and the Icare® tonometer measured IOP in a masked fashion in 100 patients. In another series of 58 patients, Icare® tonometry was performed by an inexperienced optometrist and GAT was performed by an experienced optometrist. They demonstrated that the Icare® tonometer compared reasonably well with GAT in both experienced and inexperienced hands, and concluded that the high degree of accuracy obtained in the inexperienced hands showed that it is a useful instrument for healthcare workers with limited ophthalmic experience. We previously examined whether there was a correlation in the IOP readings using the Icare® tonometer as measured by an ophthalmologist and by the patients themselves. Our findings showed that there was a moderate, but not strong, correlation between the values measured by each method (data not published). It is possible that the patients themselves had difficulty in accurately positioningthe Icare® probe toward the center of their cornea. In this present study, a correlation was found between the IOP readings obtained by the ophthalmologist and those obtained by the participants during self–examination in 81 of the 104 eyes (r = 0.674). In addition, this correlation was stronger when the position between the probe and the cornea was in better alignment. In 42 eyes in which the probe was accurately positioned toward the corneal center, the readings were strongly correlated (r = 0.884). From these findings, we theorize that with this newly developed method for self–examination by use of a modified eyeglass frame, the Icare® tonometer might produce highly reliable and also reproducible IOP self–measurements.
Previous studies have reported that IOP readings obtained by Icare® averaged 0.6–1.8mmHg higher compared with the IOP readings obtained with GAT [14–17]. Nakamura [18] compared IOP measurements obtained using Icare® with those obtained using GAT and reported a mean difference (95% limit of agreement) in IOP readings between Icare® and GAT of 1.40±4.29 mmHg. In this present study, the average of IOP using the Icare® tonometer was 1.1 mmHg higher than that measured by GAT, thus supporting the results obtained in the previous reports. Therefore, ophthalmologists need to keep this tendency in mind when using Icare® for the measurement of IOP.
In the 42 eyes in which the probe was precisely positioned toward the corneal center, we compared the IOP readings between the right and left eyes and found that the right eyes showed a stronger correlation than the left eyes. We theorize that this is may be due to the direction of the probe possibly being slightly tilted because all of the participants were right–handed, thus producing a crossbody action when measuring the left eye with their dominant right arm. To the best of our knowledge, this is the first report to describe the phenomenon that the measurement by one’s dominant arm may influence the readings of IOP when using Icare® for the selfexamination of IOP.
It should be noted that limitations associated with this present study include the small number of participants involved, the relatively younger study population compared with the average age of glaucoma patients, the fact that the participants were not glaucoma patients and that they were all right–handed, and the lack of information about each participant’s central corneal thickness (CCT) and hysteresis, which is known to strongly influenced IOP readings obtained with the Icare® [19–21]. However, Pacrou [11] reported that their findings related to the influence of CCT on IOP measurements obtained by the Icare® were unlikely to be of significance in most clinical situations, especially if the Icare® was used as a screening instrument. In their study, the Icare® over–read the GAT by 1 mmHg for every 100–μm increase in CCT measurements. They discussed that this result might be of significance in conditions in which there is marked corneal thickening (i.e., corneal edema). On the other hand, Munkwitz [22] reported that in high IOP values, measurements obtained by use of the Icare® tonometer do not correlate well with those obtained by GAT. Therefore, further studies may be needed before applying this self–examination method to glaucoma patients.
In conclusion, using our new self–examination method, the Icare® tonometer produced highly reliability IOP readings. This tonometer has the possibility of being used as a home tonometer and will be utilized clinically for the detection of diurnal changes in IOP. The new type Icare ONE®, which is specially designed for the self–examination of IOP, is soon to be released in Japan, and it has been reported that the new model Icare® produces good results [23]. The Icare® used in this present study was not specifically designed for self–examination, however, adding the simple supplementary method it was found to be reliable and highly effective for the self–examination of IOP.
Acknowledgement
The authors wish to thank John Bush for reviewing the manuscript.
References
- Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996; 80: 389-393.
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 701-713.
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 714-720.
- Landers J, Goldberg I, Graham SL. Analysis of risk factors that may be associated with progression from ocular hypertension to primary open angle glaucoma. Clin Experiment Ophthalmol. 2002; 30: 242-247.
- Kass MA. Standardizing the measurement of intraocular pressure for clinical research. Guidelines from the Eye Care Technology Forum. Ophthalmology. 1996; 103: 183-185.
- Saccà SC, Rolando M, Marletta A, Macrí A, Cerqueti P, Ciurlo G. Fluctuations of intraocular pressure during the day in open-angle glaucoma, normal-tension glaucoma and normal subjects. Ophthalmologica. 1998; 212: 115-119.
- Kontiola AI, Goldblum D, Mittag T, Danias J. The induction/impact tonometer: a new instrument to measure intraocular pressure in the rat. Exp Eye Res. 2001; 73: 781-785.
- Naruse S, Mori K, Kinoshita S. Evaluation of the pressure phosphene tonometer as a self-tonometer. Ophthalmic Physiol Opt. 2005; 25: 421-428.
- Kontiola AI. A new induction-based impact method for measuring intraocular pressure. Acta Ophthalmol Scand. 2000; 78: 142-145.
- Kontiola A, Puska P. Measuring intraocular pressure with the Pulsair 3000 and Rebound tonometers in elderly patients without an anesthetic. Graefes Arch Clin Exp Ophthalmol. 2004; 242: 3-7.
- Pakrou N, Gray T, Mills R, Landers J, Craig J. Clinical comparison of the Icare tonometer and Goldmann applanation tonometry. J Glaucoma. 2008; 17: 43-47.
- Davies LN, Bartlett H, Mallen EA, Wolffsohn JS. Clinical evaluation of rebound tonometer. Acta Ophthalmol Scand. 2006; 84: 206-209.
- Abraham LM, Epasinghe NC, Selva D, Casson R. Comparison of the ICare rebound tonometer with the Goldmann applanation tonometer by experienced and inexperienced tonometrists. Eye (Lond). 2008; 22: 503-506.
- Martinez-de-la-Casa JM, Garcia-Feijoo J, Castillo A, Garcia-Sanchez J. Reproducibility and clinical evaluation of rebound tonometry. Invest Ophthalmol Vis Sci. 2005; 46: 4578-4580.
- van der Jagt LH, Jansonius NM. Three portable tonometers, the TGDc-0, the ICARE and the Tonopen XL, compared with each other and with Goldmann applanation tonometry. Ophthalmic Physiol Opt. 2005; 25: 429-435.
- Fernandes P, Díaz-Rey JA, Queirós A, Gonzalez-Meijome JM, Jorge J. Comparison of the ICare rebound tonometer with the Goldmann tonometer in a normal population. Ophthalmic Physiol Opt. 2005; 25: 436-440.
- Martinez-de-la-Casa JM, Garcia-Feijoo J, Vico E, Fernandez-Vidal A, Benitez del Castillo JM, Wasfi M, et al. Effect of corneal thickness on dynamic contour, rebound, and goldmann tonometry. Ophthalmology. 2006; 113: 2156-2162.
- Nakamura M, Darhad U, Tatsumi Y, Fujioka M, Kusuhara A, Maeda H, et al. Agreement of rebound tonometer in measuring intraocular pressure with three types of applanation tonometers. Am J Ophthalmol. 2006; 142: 332-334.
- Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000; 44: 367-408.
- Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005; 31: 156-162.
- Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg. 2005; 31: 146-155.
- Munkwitz S, Elkarmouty A, Hoffmann EM, Pfeiffer N, Thieme H. Comparison of the iCare rebound tonometer and the Goldmann applanation tonometer over a wide IOP range. Graefes Arch Clin Exp Ophthalmol. 2008; 246: 875-879.
- Sakamoto M, Kanamori A, Fujihara M, Yamada Y, Nakamura M, Negi A. Assessment of IcareONE rebound tonometer for self-measuring intraocular pressure. Acta Ophthalmol. 2014; 92: 243-248.