Clinical Images
Austin J Clin Ophthalmol. 2014;1(5): 1022.
A Case of Dry Age-Related Macular Degeneration with Reticular Pseudodrusen
Shinji Makino*, Meri Watanabe and Hironobu Tampo
Department of Ophthalmology, Jichi Medical University, Japan
*Corresponding author: Shinji Makino, Department of Ophthalmology, Jichi Medical University, 3311-1 Yakushiji, Shimotsuke, Tochigi 329-0498, Japan
Received: April 10, 2014; Accepted: June 02, 2014; Published: June 06, 2014
Abstract
This report describes the progression of dry age-related macular degeneration with reticular pseudodrusen (RPD) over time. A 61-year-old woman who complained of unclear vision in both eyes was referred to our hospital. Her best-corrected visual acuity was 1.2 in both eyes. Funduscopic examination revealed macular atrophy surrounded by numerous RPD spread throughout the posterior pole and mid periphery of the retina in both eyes. Four years after the initial visit, her visual acuity was 0.1 in the right eye and 0.08 in the left eye. Upon further funduscopic examination, macular atrophy was found to have progressed with increased visibility of the choroidal blood vessels, and RPD were still visible around the atrophied areas. Fundus auto fluorescence imaging revealed hypo fluorescence, and near-infrared reflectance imaging detected hyper fluorescent areas in the posterior pole. Fluoresce in angiography revealed a transmission defect with a granular hyper fluorescence, and indo -cyanine green angiography showed hypo fluorescence within the lesion. Optical coherence tomography provided clear evidence of RPD above the retinal pigment epithelium. The macular thickness was reduced, the photoreceptor line was undetectable, and the choroidal signal was enhanced inside the atrophied areas in both eyes. Reduced amplitude was also noted in multifocal electroretinograms.
Keywords : Reticular pseudodrusen; Age-related macular degeneration; Optical coherence tomography
Abbreviations
AMD: Age-related Macular Degeneration; GA: Geographic Atrophy; OCT: Optical Coherence Tomography; RPD: Reticular Pseudodrusen; RPE: Retinal Pigment Epithelium.
Background
Reticular pseudodrusen (RPD) were originally classified as a type of drusen, and their appearance is somewhat similar to that of soft confluent drusen. However, their clinical features, including the sites at which they develop, their expansion and confluence over time, and their lack of fluorescence on fluoresce in or indocyanine green angiography indicate that they are not true drusen. Therefore, they are known as Pseudodrusen [1,2]. Reticular Pseudodrusen are a known risk factor for age-related macular degeneration (AMD) [2-6], which is often seen in conjunction with geographic atrophy (GA) [7,8]. Pumariega et al. [6] first described a strong association between RPD and progression to advanced AMD, and Marsiglia et al. [8] reported a strong spatiotemporal association between RPD and GA progression in the setting of dry AMD. Here, we report the case of a patient with dry AMD associated with RPD and describe the progression to advanced AMD over time.
Case Presentation
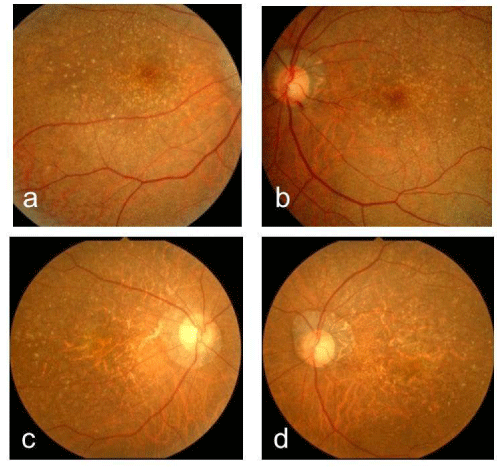
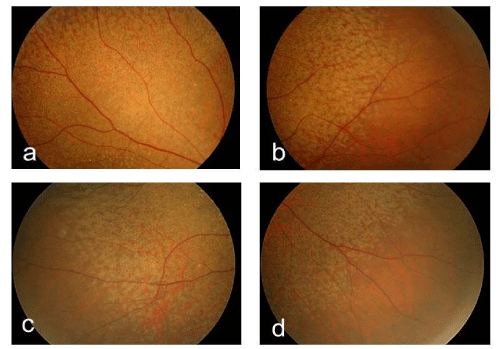
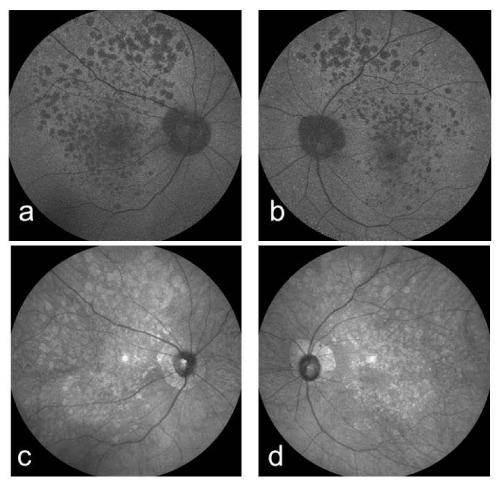
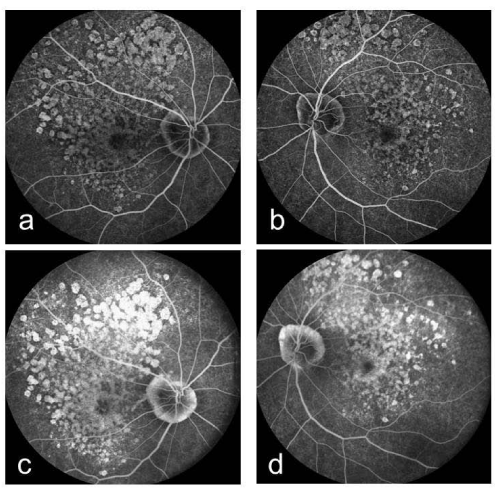
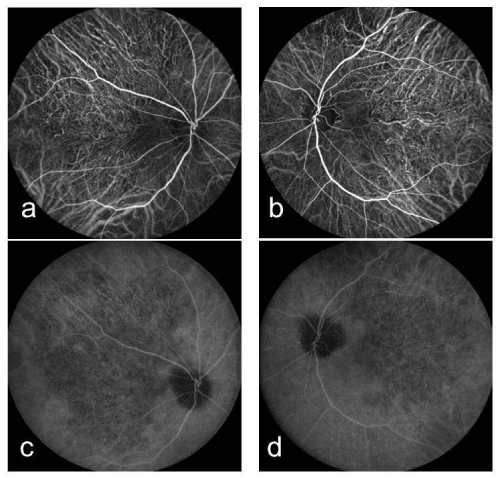
A 61-year-old woman who complained of unclear vision in both eyes was referred to our clinic at Jichi Medical University Hospital. Her best-corrected visual acuity was 1.2 in both eyes. Her personal and family history and systemic evaluation were unremarkable. However, funduscopic examination revealed macular atrophy in both eyes (Fig. 1a, b). The macular atrophy was surrounded by numerous RPD spread throughout the posterior pole and mid periphery of the retina. Four years after the initial visit, she was referred to our clinic because of progressive worsening of her visual acuity, which was 0.1 in the right eye and 0.08 in the left eye. Intraocular pressure measurements were normal. Slit-lamp examination showed cortical opacities in both lenses, and funduscopic examination revealed bilateral progressed macular atrophy with increased visibility of the choroidal blood vessels. The RPD were still visible around the atrophied areas (Fig. 2 a-d). Fundus autofluorescence (Fig. 3 a, b) imaging showed hypofluorescence and near-infrared reflectance (Fig. 3 c, d) imaging showed hyperfluorescent areas in the posterior pole. Fluorescein angiography revealed a transmission defect with a granular hyperfluorescence (Fig.4 a-d), and indocyanine green angiography hypofluorescence within the lesion (Fig. 5 a-d). Choroidal neovascularization was not detected in either eye.
Figure 1 :Right (a) and left (b) fundus photographs at the initial visit showing macular atrophy. Right (c) and left (d) fundus photographs four years after the initial visit. The atrophy had progressed with increased visibility of the choroidal blood vessels. These images also show reticular psedodrusen at the posterior pole.
Figure 2:Right fundus photographs of the superotemporal (a), inferotemporal (b), superonasal (c), and inferonasal (d) peripheral retina. These images show clearly defined reticular pseudodrusen.
Figure 3 : Right (a) and left (b) fundus autofluorescence images. The atrophy is dark and well delineated in both eyes. Right (c) and left (d) near-infrared reflectance images. The atrophy is dark and well delineated in both eyes.
Figure 4 : Right (a) and left (b) fluorescein angiography early-phase images showing a transmission defect with granular hyperfluorescence. Right (c) and left (d) fluorescein angiography late-phase images showing a transmission defect with granular hyperfluorescence.
Figure 5 : Right (a) and left (b) indocyanine green angiography early-phase images showing hypofluorescence within the lesion and no dimming of the fluorescence of the underlying larger choroidal vessels. Right (c) and left (d) indocyanine green angiography late-phase images showing hypofluorescence within the lesion and no dimming of the fluorescence of the underlying larger choroidal vessels.
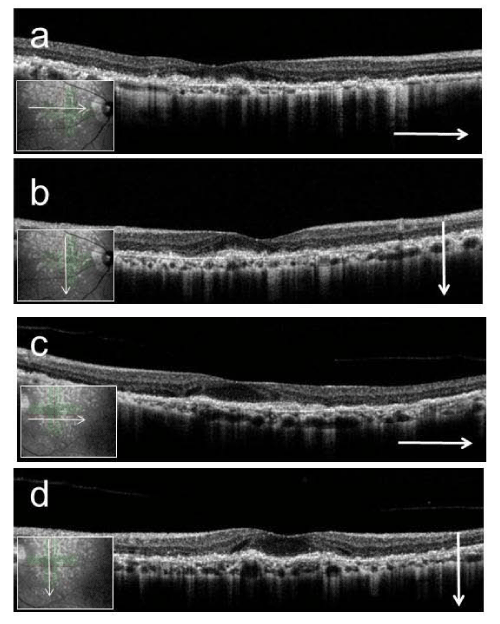
Figure 6 : Right (a, b) and left (c, d) optical coherence tomography images at the posterior pole (a, c; horizontal scan, b, d; vertical scan). Macular thickness is reduced, the photoreceptor line is undetectable, and the choroidal signal is enhanced. Hyperreflective punctate materials are visible.
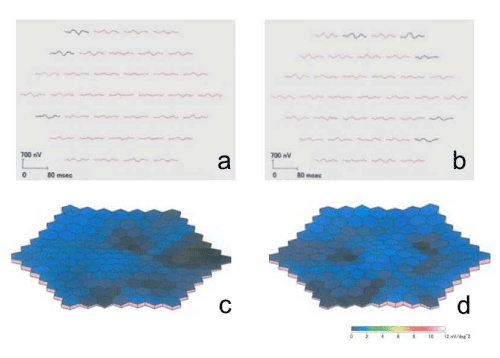
Figure 7 : Right (a) and left (b) multifocal electroretinogram response arrays showing reduced responses. Right (c) and left (d) three-dimensional plots showing reduced responses.
The Heidelberg Retina Angiograph 2 (Heidelberg Engineering, Heidelberg, Germany) was used for fundus autofluorescence, near-infrared reflectance, fluorescein angiography, and indocyanine green angiography imaging. Optical coherence tomography (OCT; RS- 3000; NIDEK, Gamagori, Japan) provided clear evidence of RPD above the retinal pigment epithelium (RPE) (Fig. 6 a-d). The macular thickness was reduced, the photoreceptor line was undetectable, and the choroidal signal was enhanced inside the atrophied areas in both eyes. Multifocal electroretinograms (LE-4100; TOMEY, Nagoya, Japan) showed reduced amplitude (Fig. 7 a-d). The patient continues to receive low vision rehabilitation.
Discussion
This report describes the progression of dry AMD with RPD to advanced AMD over time. The RPD that were spread throughout the posterior pole and the mid periphery of the retina in our subject were similar to those noted in previous descriptions [9-13]. Zweifel et al. [9] described OCT findings of RPD where the OCT scans showed collections of granular hyper reflective material above the RPE in the sub retinal space located primarily between the RPE and the boundary between the inner and outer segments of the photoreceptors (IS/OS boundary). At a more advanced stage, this material formed small mounds that broke through the IS/OS boundary. In our case, RPD were detected above the RPE, the OCT scan showed decreased macular thickness and an enhanced choroidal signal, and the IS/OS boundary line was undetectable.
Reticular pseudodrusen are a known risk factor for AMD [2-6]. In the largest series of AMD patients reported to date, Pumariega et al. [6] evaluated 271 patients with AMD to determine the risk of progression to advanced AMD conferred by RPD. The 271 subjects who completed the full 3-year study had a significantly higher rate of advanced AMD (56%) in fellow eyes with RPD at any visit than eyes without RPD (32%). In addition, Marsiglia et al. [8] reported that GA progression was more frequent in fields with RPD than in those without RPD.
Lee et al. [14] evaluated the morphological features and prevalence of accompanying late AMD based on the fundus distribution of RPD, which was classified as localized, intermediate, or diffuse based on the area in the fundus in which these were located. In their study, they found that the distribution of RPD in the 233 eyes of their 121 patients was localized in 30.9%, intermediate in 40.3%, and diffuse in 28.8%. The prevalence of accompanying late AMD was 13.9%, 13.8%, and 56.7%, respectively, for the localized, intermediate, and diffuse types, and it was significantly higher for the diffuse type. Our patient had a diffuse type RPD distribution and her condition progressed to advanced AMD over time.
Our current findings are based on a single case; however, the presence of RPD and their distribution might be important for the assessment of visual prognosis in dry AMD, because RPD are markers of high risk for the progression of AMD.
Competing Interests
The authors have no financial or proprietary interests related to this paper.
References
- Mimoun G, Soubrane G, Coscas G. Macular drusen. J Fr Ophtalmol. 1990; 13: 511-530.
- Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina. 1995; 15: 183-191.
- Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BE. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008; 145: 317-326.
- Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010; 117: 1775-1781.
- Querques G, Canouï-Poitrine F, Coscas F, Massamba N, Querques L, Mimoun G, et al. Analysis of progression of reticular pseudodrusen by spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 1264-1270.
- Pumariega NM, Smith RT, Sohrab MA, Letien V, Souied EH. A prospective study of reticular macular disease. Ophthalmology. 2011; 118: 1619-1625.
- Schmitz-Valckenberg S, Alten F, Steinberg JS, Jaffe GJ, Fleckenstein M, Mukesh BN, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 5009-5015.
- Marsiglia M, Boddu S, Bearelly S, Xu L, Breaux BE Jr, Freund KB, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013; 54: 7362-7369.
- Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010; 117: 303-312.
- Schmitz-Valckenberg S, Steinberg JS, Fleckenstein M, Visvalingam S, Brinkmann CK. Combined confocal scanning laser ophthalmoscopy and spectral-domain optical coherence tomography imaging of reticular drusen associated with age-related macular degeneration. Ophthalmology. 2010; 117: 1169-1176.
- Querques G, Querques L, Martinelli D, Massamba N, Coscas G, Soubrane G, et al. Pathologic insights from integrated imaging of reticular pseudodrusen in age-related macular degeneration. Retina. 2011; 31: 518-526.
- Fleckenstein M, Charbel Issa P, Helb HM, Schmitz-Valckenberg S, Finger RP, Scholl HPN, et al. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008; 49: 4137-4144.
- Sohrab MA, Smith RT, Salehi-Had H, Sadda SR, Fawzi AA. Image registration and multimodal imaging of reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2011; 52: 5743-5748.
- Lee MY, Yoon J, Ham DI. Clinical features of reticular pseudodrusen according to the fundus distribution. Br J Ophthalmol. 2012; 96: 1222-1226.