Research Article
Austin J Clin Ophthalmol. 2014;1(5): 1024.
TSH Receptor Antibodies as Measured in the Thyroid Stimulating Immunoglobulin (TSI) Reporter Bioassay Thyretain are not Detected in Patients with Euthyroid Graves’ Disease
Hooshang Lahooti1, Jeffrey A Houtz2, Huy A Tran3, Ilhem Al-Honchari1, Simon D Lytton2, Bernard Champion1 and Jack R Wall1*
1Department of Medicine, University of Sydney, Australia
2Department of Medicine, SeraDiaLogistics, Germany
3Department of Clinical Chemistry, Newcastle University, Australia
*Corresponding author: Jack R Wall, Department of Medicine, University of Sydney, Nepean Hospital, PO Box 63, Penrith NSW 2751, Australia
Received: April 30, 2014; Accepted: June 16, 2014; Published: June 18, 2014
Abstract
Background: The ophthalmopathy associated with Graves’ hyperthyroidism is most likely a T-cell mediated disorder, although most investigators believe that auto antibodies directed against the TSH Receptor (TSH-R) expressed on the surface of the orbital fibroblasts and pre-adipocytes are the main driver of the orbital reactions. Cases of ophthalmopathy without TSH-R binding antibodies and not closely associated with Thyroid Stimulating Immunoglobulin’s (TSI), challenge the notion that ophthalmopathy is perpetrated by TSI circulating in peripheral blood and TSI producing plasma cells resident in the orbital tissues. One way to address a possible role of TSH-R antibodies in the development of ophthalmopathy is to study patients with so-called “Euthyroid Graves’ eye Disease (EGD)”, who have the same eye disorder but with normal thyroid function and no features of thyroid autoimmunity during long term follow up.
Methods: The cell-based reporter bioassay Thyretain ™ was used to assess TSI in serum of the patients (n=50) and control subjects (n=20); The patient groups; EGD (n=13), of whom 6 were first tested early in the course of their eye disorder (< 6 months) and 7 tested ≥ 6 months after the onset of eye symptoms, Graves’ disease with (n=13), and without (n=12), ophthalmopathy and euthyroid relatives from a previously studied family having a high prevalence of thyroid autoimmunity and ophthalmopathy (WH-Fam n=12). Thyretain ™-TSI results were expressed as per cent of the sample to reference ratio (SRR %), a positive test being taken as an SRR% of > 140%.
Results: Only 3 of 13 (23 %) patients initially diagnosed with EGD tested TSI positive in one or more serum samples, and all 3 became hyperthyroid at the time of the positive test or soon after. Among the remaining 10 EGD patients who tested TSI negative, none developed thyroid autoimmunity during follow up visits 6months to 10 years after initial diagnosis. Normal subjects and euthyroid relatives from WH-Fam all tested TSI negative whereas 9 out of 12 (75%) Graves’ Hyperthyroidism (GH) and 11 out of 13 (86%) Graves’ Ophthalmopathy (GO) patients tested TSI positive.
Conclusion: These findings of positive TSI among patients with GH and GO, but not among patients with EGD with the exception of the 3 patients who developed Graves’ hyperthyroidism, strongly suggests that TSH-R antibodies cannot play a major role in the pathogenesis of what is best called endocrine or auto immuneophthalmopathy. Keywords: Ophthalmopathy; Thyroid autoimmunity; TSH receptor antibodies; Euthyroid Graves’ disease; Thyretain TM TSI reporter bioassay
Introduction
Euthyroid Graves’ Disease (EGD) is an autoimmune condition of the eye and orbital tissues in the absence of thyroid dysfunction and is presumed to be the same eye disorder that is associated with Graves’ hyperthyroidism, where it is known as “Graves’ ophthalmopathy”. Some of these patients do develop Graves’ hyperthyroidism or Hashimoto’s thyroiditis on long term follow up [1] but, in a more recent study [2], none of 7 patients developed any evidence of thyroid dysfunction or autoimmunity during up to 11 years of follow up. In these patients, Thyroid Peroxidase (TPO), Thyroglobulin (TG) and TSH receptor (TSH-R) antibodies measured as TSH-R binding antibodies (TRAb), and as Thyroid-Stimulating Immunoglobulin (TSI) measured in a cAMP reporter bioassay, were always negative [2]. The pathogenesis of Graves’ ophthalmopathy, which is presumed to be the same eye disorder but associated with hyperthyroidism, is best explained by the action of T-lymphocytes and antibodies that target auto antigens shared between the thyroid and orbit muscle tissue, such as the TSH-R expressed on the orbital pre-adipocytes and fibroblasts [3-6], the main candidate antigen. However, the TSH-R is expressed in non-thyroid tissues as well and anti-TSH-R antibodies are not detected in all patients with Graves’ ophthalmopathy at the time of diagnosis [7], nor in patients with Hashimoto’s thyroid it is with mainly upper eyelid signs and symptoms [8,9]. For this reason, the conspicuous absence of TSH-R antibodies upon multiple visits of patients with EGD implies that anti-TSH-R antibodies are neither necessary nor sufficient for the pathogenesis of ophthalmopathy associated with thyroid autoimmunity.
TSI by Thyretain ™ -reporter bioassay (Thyretain-TSI) were previously shown to be functional indicators of the activity and severity of Graves’ ophthalmopathy; Furthermore, the combination of negative TRAb and positive Thyretain-TSI is closely associated with ophthalmopathy (Lytton et al [10-12] while patients testing positive for TRAb and negative for Thyretain-TSI had Graves’ disease without orbitopathy [12]. However, Thyretain-TSI is also found in newly diagnosed Graves’ disease patients showing no clinical signs of eye disease. In the present study we have used the Thyretain ™ bioassay to assess the TSI levels in sera from 13 patients with EGD, including several seen on repeated visits within a few months of the onset of symptoms. The TSI levels of these EGD patients were compared with normal controls and with three other patient groups; hyperthyroid Graves’ disease patients with and without ophthalmopathy, and 12 of their euthyroid relatives, belonging to single family with a high prevalence of thyroid autoimmunity.
Clinical Subjects and Methods
Clinical subjects
We studied sera from; 1) 13 patients with euthyroid Graves’ disease, 9 females and 4 males aged 43-70 (mean age 56years). Multiple serum samples from 6 of the patients and single samples from 7 patients were tested in single Thyretain TSI reporter bioassays. All 13 patients were diagnosed as “euthyroid Graves’ disease” at the time of the initial blood sample. Although 3 patients (nos. 1,2 and 3 Table 1), subsequently converted to Graves’ hyperthyroidism (2 patients) or had developed Graves’ disease many years earlier (1 patient).
Patient
number
Age/gender
Ethnicity
Thyroid function1
Thyroid ultrasound
Histological assessment2
fT4
TSH
1
55/F
Caucasian
12.3
0.74
Normal
NP3
2
43/M
Caucasian
Normal
Normal
NP
NP
3
70/F
Caucasian
Normal
Normal
NP
NP
4
54/F
Caucasian
14
0.42
Normal
NP
5
50/F
Asian
9.9
0.84
Not performed
NP
6
60/F
Asian
14
0.69
One small nodule else normal
NP
7
63/F
Caucasian
12.6
1.1
Normal
NP
8
53/F
Malay
16
1.91
One large nodule, one small nodule else normal
Follicular cell adenoma, no
small lymphocytes
on FNAB or at thyroidectomy
9
49/F
Caucasian
13
0.97
Several big nodules and overall colloid features
Colloid goitre, no lymphocytes at thyroidectomy
10
51/M
Caucasian
9.9
Normal
Small nodules
NP
11
63/M
Caucasian
13.7
0.63
Normal
NP
12
65/M
Caucasian
15.2
0.88
NP
NP
13
58/F
Caucasian
Normal
Normal
NP
NP
Table 1: Demographics, thyroid function and thyroid ultrasound findings at the first visit in thirteen patients with Euthyroid Graves’ disease.
2) 16 members of a single family with a high prevalence of Graves’ disease (WH-Fam) [13], comprising 4 probands, 3 females and one male and 12 first or second degree euthyroid relatives, 8 females and 4 males, aged 13 -76 (mean age 40 years), some of whom were studied previously [13].
3) 24 patients with Graves’ disease, including 3 of the probands from WH-Fam with Graves’ disease, 20 females and 5 males aged 16 – 77 (mean age 51 years) of whom 10 females and 3 males aged 38 - 77 (mean age 57 years) had ophthalmopathy. Blood samples were taken at various times in these patients, usually soon after diagnosis and at various times during anti thyroid drug therapy. Most patients had been TSH-R antibody positive in the TRAb and cAMP based TSI assays on at least one occasion.
4) 20 normal subjects comprising; 15 males aged < 30 whose sera had been previously used to develop a reference range for an ELISA to measure CASQ1 and collagen XIII antibodies and 5 other subjects, 4 females and one male, aged 28 – 50 (mean age 32 years).
The diagnosis of the various thyroid disorders was made according to conventional clinical parameters and confirmed by serum freeT4 (fT4), free T3 (fT3) and TSH measurements and from anti - TG and anti - TPO antibody tests and, in many cases, real time thyroid ultra sonography. Local Ethical Committee approval was received for this retrospective study and informed consent was not required.
Eye assessment
The ophthalmopathy was assessed as; i) Nunery type 1 (without restrictive myopathy) or type 2 (with restrictive myopathy) [14]. ii) a modified Clinical Activity Score (CAS) (0-12) of Mourits et al. [15] which is a measure of disease activity iii) Werner’s NOSPECS classes [16] and 4) upper eyelid Margin-Reflex Distance (MRD) which is the distance between the centre of the pupillary light reflex and the upper eyelid margin with the eye in primary gaze, as a measure of eyelid retraction; an MRD of > 5mm is taken as significant UER. The degree of proptosis (mm) was measured using a Hertel exophthalmometer where a positive reading was defined as > 18mm in either eye or > 2 mm difference between the eyes. For the purpose of the study “ophthalmopathy” was taken as a NOSPECS class ≥ 2, regardless of the CAS.
Thyretain ™ TSI reporter bioassay
The Thyretain ™-TSI cAMP luciferase reporter bioassay described previously [10-12] was used to assess TSI. Briefly, test serum samples and four controls, consisting of reference standard bovine TSH (bTSH), normal serum, positive TSI serum and cells alone, were tested in triplicate. Results were expressed as per cent of the Sample to Reference Ratio (SRR %). The cut-off of the Thyretain-TSI reporter % of the Sample to Reference Ration (SRR%) was historically established as 2SD above the reference luminescence, which is set to SRR 100% for each plate, a positive test being taken as an SRR% of > 140%.
Other tests
Plasma fT4, fT4 and TSH levels and serum TPO and TG antibodies were measured by Barratt and Smith Pathology, Sydney, Australia, according to the manufacturers’ instructions.
Statistical analysis
Differences in the TSI levels between the various Graves’ disease patient groups and the control subjects were assessed using the Mann-Whitney test for non parametric data; a p value of <0.05 was taken as statistical significance.
Results
We used a new cell-based bioassay for Thyretain-TSI that has been shown to measure those TSH-R antibodies that are functional indicators of the existence, activity and severity of Graves’ ophthalmopathy. We tested serum from 13 patients who initially presented for eye muscle antibody testing with the diagnosis of EGD. Six of the 13 EGD patients were first tested early (< 6 months) in the course of their eye disorder and the remaining 7 patients were tested 6 months or more after the onset of eye symptoms (Table 3). The eye disease at the first visit ranged from moderately severe to severe; NOSPECS range 2-4, mean 3.4, and 7 patients had eye muscle dysfunction (Nunery 2) and active; CAS range 1-6, mean 3.5, The time between onset of symptoms and the first TSI test varied from 4-48 months (mean 14.2 months) and duration of follow up time since the onset of eye symptoms ranged from 8 months to 12 years (Table 4).
Twenty-four patients with Graves’ disease with (n=13) and without (n=12) ophthalmopathy and 12 euthyroid relatives from WH-Fam with a high prevalence of thyroid autoimmunity. Graves’ patients were included as a single group regardless of the time since diagnosis, duration of any anti-thyroid drug therapy and presence or not of ophthalmopathy and included 3 of the probands from WH-Fam [WH-25, WH-30, and WH-18 (13)]. The severity and activity of the ophthalmopathy were similar to those of patients with EGD. A positive Thyretain-TSI test was taken as SRR% > 140%, established in preliminary studies by Lytton [10-12]. The focus of the present study is the measurement of TSH-R antibodies in a Thyretain-TSI reporter bioassay in patients with EGD.
Demographics, thyroid function and thyroid ultrasound findings at the first visit in the patients with EGD are summarised in Table 1 and eye findings, smoking status and treatment are summarised in Table 2. In table 3 are shown serum thyroid antibodies, other auto antibodies and personal and family history of thyroid and other autoimmune disorders in these patients. Serum T4, T3 and TSH levels were always normal in these patients, and serum TPO and TG antibody tests were always negative (Table 3). Where measured, antinuclear antibody tests were negative in all but one patient. One other patient had a positive Gastric Parietal Cell (GPC) antibody test and 3 had positive tests for the Flavoprotein (Fp) (the “64 kDa protein”) antigen. TSH-R antibodies measured in the TRAb assay were negative in all patients except on one occasion only, and corresponding to a positive Thyretain-TSI test, in patient no. 2 (see later) while thyroid-stimulating antibodies measured in a conventional cAMP based immunoassay were negative in all subjects tested on all occasions except in patient no. 4 who had a positive test - but negative TRAb and Thyretain-TSI - on one occasion (results not shown).
Patient number
Smokes
Eye Signs
Orbital CT findings
Treatment
NOSPECS Class 3
CAS1
Nunery Type4
UER2
1
No
4
1
2
Yes
EOM 5 volumes increased
Prednisolone 7.5 mg
2
No
4
4
2
No
NP 6
Nil
3
No
2
3
1
No
NP
LT4 @ time of second blood test
4
Yes
2
3
1
No
NP
Nil
5
No
3
5
1
Yes
Normal EOM
Nil
6
No
2
3
1
No
Normal on two occasions 1 yr. apart
Prednisolone (50mg/day) + steroid eye drops
7
No
4
4
2
Yes
Enlarged EOM5
Nil
8
No
4
4
2
No
Enlarged EOM
Prednisolone 25mg
9
No
3
4
1
No
Minimal EOM
Nil
10
No
4
4
2
No
Enlarged EOM on left only
Nil
11
No
4
6
2
Yes
EOM volumes increased
Nil
12
No
4
1
2
No
Enlarged left inf. rectus muscle only
Nil
13
No
4
4
2
No
NP
Pulse MP. Earlier she was hypothyroid for years but not treated
Table 2: Eye findings, smoking status, orbital CT findings and treatment in 13 patients with Euthyroid Graves’ disease at the first visit.
Patient
Number
Thyroid antibodies1
Other autoantibodies2
Personal history of thyroid or other autoimmunity
Known family of thyroid or other autoimmunity
TPO
TG
ANA
Other
1
<20
<20
Nag
NT3
Nil
“Thyroid disease”
2
<20
<20
NT
Fp
Not known
Nil
3
<20
<20
NT
NT
Not known
Nil
4
<20
<20
NT
NT
Not known
Nil
5
<20
<20
Nag
NT
Nil
Rheumatoid Arthritis
6
<20
<20
Nag
GPC
Vitiligo
Nil
7
<20
<20
Nag
Fp
Nil
Hashimoto's
thyroiditis
8
<20
<20
Nag
Fp
Nil
Nil
9
<20
<20
Nag
NT
Nil
Nil
10
<20
<20
NT
NT
Nil
Nil
11
<20
<20
Pos 1/80
NT
Not known
Nil
12
<20
<20
NT
NT
Not known
Nil
13
<20
<20
NT
NT
Not known
Nil
Table 3: Thyroid antibodies, other auto antibodies and personal and family history of thyroid. And other autoimmune disorders in 13 patients with Euthyroid Graves’ disease at the first visit.
Thyretain-TSI tests were always negative in 10 patients with EGD but positive, at one test only, in 3 patients (nos. 1, 2 and 3, Table 4). Four of the patients are described in more detail; patient no. 1 was referred as “EGD” for eye muscle antibody testing and had a strongly positive Thyretain-TSI test; however, it turned out that she had developed Graves’ hyperthyroidism soon after the test. Patient no. 2 had been managed by his ophthalmologist and endocrinologist for many years as “EGD” and also had a strongly positive test in his first serum sample (an earlier TRAb test had beennegative), just before he too converted to Graves’ hyperthyroidism. His Thyretain-TSI tested negative 4 months later (Table 4). Patient no. 3 had a 4 months history of “EGD” and a negative Thyretain-TSI test at the time of her first TSI test. Her second TSI test, 6 months later, was strongly positive and corresponded with the development of Graves’ hyperthyroidism soon after. She was then treated with I131, becoming hypothyroid at the time of her third, negative, test another 6 months later. Finally, patient no. 13 was diagnosed with Graves’ disease 18 years before her first TSI test at which time she was treated with anti thyroid medication. She became hypothyroid 8 yr. later despite not being treated with 131I or thyroidectomy. Twelve months before her TSI test she developed severe, active ophthalmopathy, requiring treatment with IV methyl prednisolone and oral steroids. At the time of her first TSI test her ophthalmopathy was in remission and stable and she was euthyroid. At that time TRAb and TG antibody tests were negative but TPO antibody was positive (titre 475), and her Thyretain-TSI test was negative (Table 4).
Patient Number
Duration (months): ophthalmopathyonset to first TSI test
Serum 1TSI-Thyretain level (SRR %)
1
4
357%
2
48
332%
52
138%
3
4
90%
10
405%
16
67%
4
5
108%
5
18
43%
6
24
60%
40
66%
60
42%
66
31%
72
35%
78
52%
84
48%
90
58%
96
91%
7
30
44%
32
45%
46
50%
51
42%
60
44%
8
12
49%
13
64%
15
107%
21
85%
27
48%
33
75%
39
25%
45
68%
51
51%
57
57%
60
68%
66
49%
9
18
43%
10
8
33%
11
5
43%
8
44%
12
3
75%
13
6
45%
Table 4: Serum TSI by cAMP assay and TSI-Thyretain levels in 13 patients with Euthyroid Graves’ disease at each clinic visit.
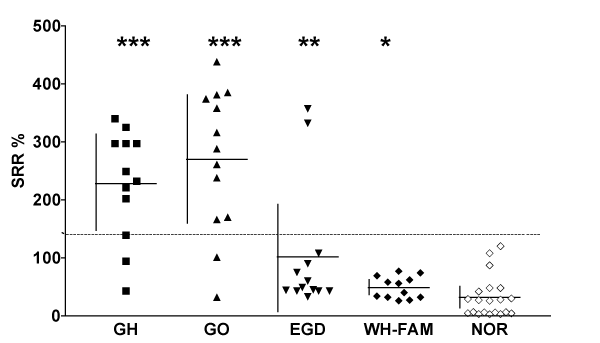
All other tests in the other 10 patients were always negative and none of these patients have developed thyroid autoimmunity during follow up of from 6 months to 12 years. Thyretain-TSI levels at the first blood test only and mean (+/- SD) for the group are shown in Figure 1. Also shown in fig. 1 are initial Thyretain-TSI levels in patients with Graves’ hyperthyroidism, Graves’ ophthalmopathy euthyroid members of WH-Fam and normal subjects. Tests were negative in all euthyroid relatives in WH-Fam and in all normal’s (Figure 1). The mean (+/- SD) SRR% for patients with EGD, 95.2 (+/- 114) was just significantly different from that for the normal’s [31.8 (+/- 35) SRR%, Mann –Whitney test, p = 0.02] because of the two of the 3 patients with positive tests, who had converted to Graves’ hyperthyroidism at this time, as discussed above. Mean (+/- SD) SRR% for patients with Graves’ hyperthyroidism, with (270 +/- 113 SRR %), and without (228 +/- 94 SRR%], ophthalmopathy, were both significantly different from that for normal’s (p < 0.001, p < 0.001 respectively) but not from each other (p = NS). although levels tended to be higher in patients with severe and active ophthalmopathy (results not shown). Mean (+/- SD) SRR% for WH-Fam relatives (48 +/- 19 SRR %) was also significantly different from the normal’s (p = 0.02). We did not study the relationship between Thyretain-TSI levels and various parameters of the severity and activity (CAS, NOSPECS classes, Nunery class) of the eye disease. TRAb tests were always negative when TSI tests were negative and positive TRAb correlated with positive TSI in – out of the – patients with EGD in whom this test was performed (results not shown).
Figure 1 :Distribution of Thyroid Stimulating Immunoglobulin (TSI) levels in the different Graves Disease patients compared with normal controls.TSI levels represented as SRR% Graves’ disease with (GO, n=13) and without (GH, n=12) ophthalmopathy, Euthyroid Graves’ Disease (EGD, n=13), euthyroid relatives from a single family having high prevalence of Graves’ disease and ophthalmopathy (WH-Fam, n=12) and normal subjects, as controls (NOR, n=20). The manufacturers cut-off of positive TSI, SSR% = 140% is indicated by dotted line. *** p< .0001,* p = 0.02 as determined using Mann Whitney tests.
Discussion
Graves’s ophthalmopathy is a complex disorder because one must explain the unique link between the orbital inflammation and thyroid autoimmunity. One commonly held view is that the TSH-R antibodies that cause Graves’ hyperthyroidism also cause the ophthalmopathy by cross-reacting with the TSH-R in the orbital connective tissue [3-6,17]. It is true that TSH-R antibody levels tend to be higher in patients with ophthalmopathy and very high in those with severe active disease [18] and that the hyperthyroidism and eye signs tend to occur together when TSH-R antibody levels are first detected [19]. However, there are some situations where these antibodies are not closely associated with ophthalmopathy and the overall evidence is mainly circumstantial [7]. One way to address a possible role of TSH-R antibodies in the development of “endocrine” or “autoimmune” ophthalmopathy is to study patients with so-called “Euthyroid Graves’ Disease (EGD)”, who have the same eye disorder but with normal thyroid function and no evidence for thyroid autoimmunity on long term follow up. If they become hyperthyroid – as did 3 of our patients - they are no longer considered to have EGD, but Graves’ disease. We have measured TSH-R antibodies using a new and sensitive bioassay, which is closely linked to ophthalmopathy in patients with Graves’ disease, the Thyretain- TSI reporter bioassay. To summarise the main results; Thyretain- TSI tests were strongly positive in the great majority of patients with Graves’ hyperthyroidism and Graves’ ophthalmopathy, as expected, but negative in all normal subjects and in all euthyroid patients from a family with a high prevalence of ophthalmopathy and thyroid autoimmunity. Thyretain-TSI tests were also positive in 3 out of 13 patients with EGD, but all 3 had developed Graves’ hyperthyroidism at or soon after the positive test and tests were negative in the other 10 patients at all clinic visits over a follow up period of up to 12 years.
The notion of classes of TSH-R antibodies with different actions namely, stimulating, binding and blocking, some of which may be implicated in the pathogenesis of the ophthalmopathy associated with Graves’ hyperthyroidism, and others the cause of the hyperthyroidism, is interesting and the focus of intense study. For example, one group [20] has claimed that only blocking TSH-R antibodies are linked to the ophthalmopathy of Graves’ disease. TSH-R blocking antibodies are presumed to be different from the stimulating antibodies measured in older cAMP driven assays and the TSI measured here in the Thyretain - TSI reporter bioassay. Lytton et al [10-12] showed that antibodies detected in the Thyretain-TSI reporter assay were closely linked to the ophthalmopathy in patients with Graves’ disease whereas binding TSH-R antibodies were associated with the hyperthyroidism. We did not show a close relationship between positive Thyretain-TSI and ophthalmopathy in our patients with Graves’ disease, but this may reflect the fact that the patients were unselected for duration and severity of the hyperthyroidism or severity or activity of any associated ophthalmopathy and most patients, with or without ophthalmopathy, had high Thyretain-TSI levels.
The key finding from our study is that Thyretain-TSI tests were always negative in patients with EGD unless they had converted to Graves’ hyperthyroidism. In our series, only 3 patients have so far converted after up to 12 years follow up and it is not clear whether any others will develop Graves’ disease in the future. In other words, the ophthalmopathy and hyperthyroidism appear to be quite separate organ specific autoimmune disorders, TSI as measured in the Thyretain ™ -reporter bioassay, being associated with hyperthyroidism, but not ophthalmopathy in patients initially diagnosed with “Euthyroid Graves’ Disease”. However, we do need to measure TSH-R blocking antibodies in our patients with EGD, even though it seems unlikely that these antibodies would be detected when Thyretain-TSI and TRAb tests are only positive in those patients who have developed Graves’ hyperthyroidism.
In conclusion, the finding of positive Thyretain -TSI tests in patients with Graves’ hyperthyroidism and Graves’ ophthalmopathy, but not in patients with EGD until they developed Graves’ hyperthyroidism (which occurred in only 3 of our 13 patients studied for up to 12 years) suggests that TSH-R antibodies, regardless of how they are measured, cannot play a major role in the pathogenesis of what is best called “autoimmune” or “endocrine” ophthalmopathy [22]. A pathogenic role of other antibodies in ophthalmopathy associated or not with Graves’ hyperthyroidism, such as those targeting collagen XIII, calsequestrin [23,24] or the 64-kDa protein [25], should now be taken more seriously.
Acknowledgment
This research was supported by grants from Nepean Blue Mountains Local Health District and the Nepean Medical Research Foundation. We thank Drs. A Maloof, L Kowal, L Wallman, D Srivarstara and J Green field for supplying serum samples and clinical details from their patients. Dr J Houtz for performing the Thyretain-TSI tests.
References
- Salvi M, Zhang ZG, Haegert D, Woo M, Liberman A, Cadarso L, et al. Patients with endocrine ophthalmopathy not associated with overt thyroid disease have multiple thyroid immunological abnormalities. J Clin Endocrinol Metab. 1990; 70: 89-94.
- McCorquodale T, Lahooti H, Gopinath B, Wall JR. Long-term follow-up of seven patients with ophthalmopathy not associated with thyroid autoimmunity: heterogeneity of autoimmune ophthalmopathy. Clin Ophthalmol. 2012; 6: 1063-1071.
- Eckstein AK, Plicht M, Lax H, Neuhäuser M, Mann K, Lederbogen S, et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves' ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006; 91: 3464-3470.
- Paschke R, Vassart G, Ludgate M. Current evidence for and against the TSH receptor being the common antigen in Graves' disease and thyroid associated ophthalmopathy. Clin Endocrinol. 1995; 42: 565-569.
- Bahn RS. Clinical review 157: Pathophysiology of Graves' ophthalmopathy: the cycle of disease. J Clin Endocrinol Metab. 2003; 88: 1939-1946.
- Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves' ophthalmopathy. Thyroid. 2002; 12: 855-860.
- Wall JR. The TSH-Receptor and Thyroid-Associated Ophthalmopathy-a Convenient Hypothesis with too many Exceptions to be true. Int J Endocrinol Metab. 2007; 5: 49-51.
- Tjiang H, Lahooti H, McCorquodale T, Parmar KR, Wall JR. Eye and eyelid abnormalities are common in patients with Hashimoto's thyroiditis. Thyroid. 2010; 20: 287-290.
- Gopinath B, Ma G, Wall JR. Eye signs and serum eye muscle and collagen XIII antibodies in patients with transient and progressive thyroiditis. Thyroid. 2007; 17: 1123-1129.
- Lytton SD, Li Y, Olivo PD, Kohn LD, Kahaly GJ. Novel chimeric thyroid-stimulating hormone-receptor bioassay for thyroid-stimulating immunoglobulins. Clin Exp Immunol. 2010; 162: 438-446.
- Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves' orbitopathy. J Clin Endocrinol Metab. 2010; 95: 2123-2131.
- Lytton SD, Kahaly GJ. Bioassays for TSH-receptor autoantibodies: an update. Autoimmun Rev. 2010; 10: 116-122.
- Ardley M, McCorquodale T, Lahooti H, Champion B, Wall JR. Eye findings and immunological markers in probands and their euthyroid relatives from a single family with multiple cases of thyroid autoimmunity. Thyroid Res. 2012; 5: 4.
- Nunery WR, Martin RT, Heinz GW, Gavin TJ. The association of cigarette smoking with clinical subtypes of ophthalmic Graves' disease. Ophthal Plast Reconstr Surg. 1993; 9: 77-82.
- Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. Br J Ophthalmol. 1989; 73: 639-644.
- Werner SC. Classification of the eye changes of Graves' disease. Am J Ophthalmol. 1969; 68: 646-648.
- Weetman AP. Graves' disease. N Engl J Med. 2000; 343: 1236-1248.
- Gerding MN, van der Meer JW, Broenink M, Bakker O, Wiersinga WM, Prummel MF. Association of thyrotrophin receptor antibodies with the clinical features of Graves' ophthalmopathy. Clin Endocrinol (Oxf). 2000; 52: 267-271.
- Burch HB, Wartofsky L. Graves' ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993; 14: 747-793.
- Won Bae Kim, Hyun Kyung Chung, Young Joo Park, Do Joon Park, Kazuo Tahara, Leonard D. Kohn, et al. The Prevalence and Clinical Significance of Blocking Thyrotropin Receptor Antibodies in Untreated Hyperthyroid Graves' disease Thyroid. 2000; 10: 579-586.
- Schott M, Minich WB, Willenberg HS, Papewalis C, Seissler J, Feldkamp J, et al. Relevance of TSH receptor stimulating and blocking autoantibody measurement for the prediction of relapse in Graves' disease. Horm Metab Res. 2005; 37: 741-744.
- Wall JR. Thyroid function. Pathogenesis of Graves ophthalmopathy--a role for TSH-R? Nat Rev Endocrinol. 2014; 10: 256-258.
- Gopinath B, Ma G, Wall JR. Eye signs and serum eye muscle and collagen XIII antibodies in patients with transient and progressive thyroiditis. Thyroid. 2007; 17: 1123-1129.
- Lahooti H, Parmar K R, Wall JR. Pathogenesis of Thyroid Eye Disease: important role of autoimmunity against calsequestrin and collagen XIII. A review. ClinOphthalmol. 2010; 14: 417-425.
- Salvi M, Miller A, Wall JR. Human orbital tissue and thyroid membranes express a 64 kDa protein which is recognized by autoantibodies in the serum of patients with thyroid-associated ophthalmopathy. FEBS Lett. 1988; 232: 135-139.
Citation: Lahooti H, Houtz JA, Tran HA, Al-Honchari I, Lytton SD, et al. TSH Receptor Antibodies as Measured in the Thyroid Stimulating Immunoglobulin (TSI) Reporter Bioassay Thyretain are not Detected in Patients with Euthyroid Graves’ Disease. Austin J Clin Ophthalmol. 2014;1(5): 1024. ISSN: 2381-9162