Review Article
Austin J Clin Ophthalmol. 2014;1(7): 1033.
Idiopathic Intracranial Hypertension (IIH): Review & Updates
Marlon Demeritt, Diana Shechtman* and Sherrol Reynolds
Department of Ophthalmology, Nova Southeastern University, USA
*Corresponding author: Diana Shechtman, Department of Ophthalmology, Nova Southeastern University, USA
Received: August 20, 2014; Accepted: September 17, 2014; Published: September 19, 2014
Abstract
Idiopathic Intracranial Hypertension (IIH) is a neurological condition characterized by elevated intracranial pressure in the absence of underlying conditions, resulting in bilateral optic nerve head edema. A better understanding of the condition, including associated risk factors, clinical presentation and the use of diagnostic modalities is essential in order to derive to a proper diagnosis. Current information also provides new treatment options for the management of IIH.
Keywords: Papilledema; Idiopathic intracranial hypertension; Pseudotumorcerebri; Benign intracranial hypertension; Optic neuropathy
Introduction
Idiopathic intracranial hypertension, also known as pseudotumorcerebri (PTC) orbenign Intracranial Hypertension (BIH) is a neurological disease of unknown etiology [1]. It is characterized by elevated intracranial pressure in the absence of underlying conditions, typically resulting in bilateral optic nerve head edema. If left untreated, IIH can result in irreversible loss of vision. Advances in diagnostic modalities and treatment options have improved the outcome of IIH. This paper will provide an update on the condition, diagnosis and management of IIH illustrated through a case presentation.
IIH predominantly affects obese women in their childbearing age. The variability is the clinical presentation may add to a diagnostic challenge. Numerous risk factors ranging from medications, obstructive sleep apnea, venous sinus thrombosis, anemia, and vitamins can contribute to the condition. Table 1 lists the variable risk factors, including medications that have been associated with IIH [2].
Obesity
Nalidixic Acid
Tetracycline antibiotics (particularly minocycline)
Nitrofurantoin
Hypervitaminosis A
Oral Contraceptives
Retinoid drugs
Levonorgestrel
Cyclosporine
Danaxol
Lithium
Tamoxifen
Obstructive sleep apnea
Venous sinus thrombosis
Table 1: Risk factors for IIH.
Signs and symptoms of IIH may include [3-7] but are not limited to headaches, nausea, vomiting, vision loss, impaired visual fields, photopsias, intermittent diplopia, transient visual obscuration, pulsatile tinnitus (described as ringing in the ear) [7] shoulder or arm pain and papilledema [8]. Papilledema is by far the most common sign observed in patients with IIH associated with disruption to the axoplasmic flow in the optic nerve due to elevated cerebrospinal fluid pressure in the subarachnoid space around it. Papilledema is described as the appearance of elevated swollen bilateral optic nerves in a patient with IIH. Peripapillary hemorrhages, CWS, exudates, and macular edema may also accompany the presenting papilledema. Paton’s lines or chorioretinal folds are often observed in association with papilledema. In the presence of papilledema, visual field defects (VFDs) [9] may vary. The most commonly VFD includes increase of the blind spot. Unlike other neuropathies, there is no associated Afferent Pupillary Defect (APD) or dyschromatopsia.
Although Transient Visual Obscurations (TVOs) may accompany the presenting papilledema, visual acuity is not typically compromised. TVOs are described as brief episodes of monocular or binocular vision loss. TVOs are precipitated by postural changes and valsalva maneuvers. Other associated findings may include isolated abducens nerve (cranial nerve 6 “VI”) palsies presumably related to compression of the nerve as it exits Dorello’s canal [10]. Cranial nerve 6th palsy is attributed to nerve traction from increased intracranial pressure. This results in horizontal diplopia which commonly shows bilateral involvement [10]. Paresis of other cranial nerves (such as 3rd, 4th & 7th) are extremely rare but have been documented in the literature [1].
With regards to symptomology, headaches are the most common symptoms reported [11-15]. The associated headaches are directly correlated to the increased intracranial pressure, hence they are worse upon awaking and exacerbated by maneuvers that increase intra-cranial pressure. These are holocranial, involving the entire cerebrum. The headaches are described are gradually increasing in intensity and commonly depicted as pulsating in nature. Improvement of headaches is often noted with a decrease in intra cranial pressure [14].
IIH was initially characterized by headaches, transient visual obscuration and concomitant papilledema. A more current definition includes the following characteristics [15]:
- Symptoms and signs related to rise in intracranial pressure. These may include headache, nausea, vomiting, pulsatile tinnitus, transient visual obscurations and/or papilledema
- No associated localizing signs, with the exception of a sixth nerve palsy
- No underlying cause associated with the rise of the intracranial pressure (ICP). This may be verify by the presence of an unremarkable neuro imaging scan (brain MRI)
- Cerebrospinal Fluid (CSF) opening pressure of greater than 25 cm (250mm) H2O comprised of normal CSF composition & no associated secondary findings.
- No other alternative explanation for the raised ICP.
Dilated fundus exams, visual field testing, neuro imaging studies and lumbar puncture have routinely been used as the ophthalmological and neurological tests in the evaluation and management of patients with IIH. [16]. Dilated fundus exams allows for the visualization of the optic disc. Careful assessment of the margins for blurred disc margins and swelling of the nerve fiber layer are important in the evaluation. Visual field testing allows us to identify the visual field defects most commonly associated with papilledema. And, neuroimaging studies assist us in ruling out other causes of elevated intracranial pressure besides idiopathic causes. Given nerve swelling is often associated with IIH; the use of OCT has recently been shown rather valuable in the diagnosis and monitoring of the condition.
Conventional treatment has included both weight loss and the use of Diamox, a common prescribed diuretic. Yet, given the increase adverse affect of Diamox and the fact that it is not always well tolerated by patients, the efficacy of newer medications are being researched in the treatment of IIH [17,18].
Case Presentation
A 55-year old white female presented for an exam with complaints of bilateral transient vision loss, described as blurred glared vision associated with postural changes. She also reported ringing in her ears, dizziness, numbness of her right arm, & left leg weakness. Medical history was positive for hypertension, which was treated with a b-blocker. She did not report taking any other medications.
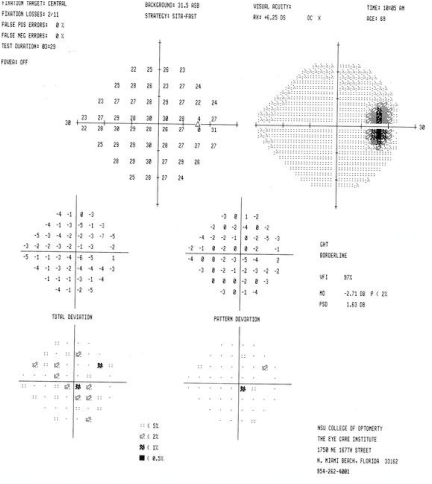
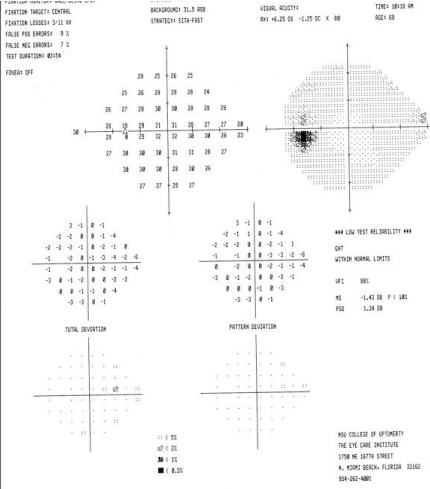
Ocular examination revealed best corrected visual acuities of 20/20 OD, OS. Blood pressure was 130/75 mmHg RAS. Extra ocular motilities, confrontation visual field, and cover test evaluation were all normal. There was no APD noted for either eye. Color vision was normal for both eyes, using the Ishihara color plates. Humphrey 24-2 VF testing revealed and increased blind spot of the right eye & an essential unremarkable VF OD (Figure 1).
Figure 1a : VF OD.
Figure 1b : VF OS.
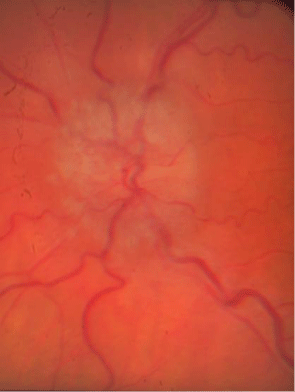
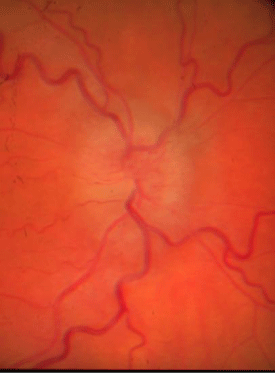
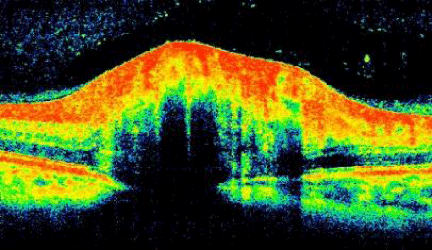
Anterior segment evaluation revealed no pathology. Goldmann applanation tonometry measured intraocular pressures of 14 mmHg in both eyes. Dilated examination revealed bilateral asymmetrical (OD>OS) optic nerve elevation & swelling with associated Paton‘s line and a cup/disc ratio of 0.15/0.15 both eyes (Figure 2). The right eye’s corresponding OCT revealed a smooth contour elevation with the presence of a lazy-V (Figure 3).
Figure 2a : OD swollen nerve.
Figure 2b : Swollen nerve OS.
Figure 3 : OCT OD.
Given the atypical presentation, a complete medical evaluation, including CBC (complete blood count) was recommended in addition to alumbar puncture and MRI was ordered. IIH was confirmed with an associated elevated lumbar puncture of over 250mmH2O in the absence of an abnormal brain MRI and an abnormal medical examination. Acetazolamide treatment was initiated for a week &the taper for the next week, Topamax® (topiramate) was added once the Diamox was tapered. The patient failed to return to clinic and was lost to follow up.
Discussion
Updates in clinical evaluation & treatment of IIH
The most common presenting clinical sign of IIH is papilledema, described as bilateral optic nerve head swelling or elevation. There are a number of associated symptoms but given the fact that not all IIH symptoms may be present, in association with papilledema poses a diagnostic challenge. Among the most common differential diagnosis for papilledema is pseudo-papilledema, associated with optic nerve drusen.
With the advent of OCT, the associated papilledema has been further analyzed helping in differential diagnostic and may be used to monitor the efficacy of treatment [19,20]. Johnson et al evaluated the clinical features that differentiating optic nerve edema and optic nerve drusen [18]. In cases of edema, the OCT finding demonstrated a smooth internal contour and with increase thickest near the optic nerve head that gradually tapers. An increase in the peripapillary retinal nerve fiber layer (RNFL) may be noted; in addition a “lazy V” pattern was also described (as seen in our patient). This was characterizing by a separation of the neurosensory retina for the RPE, associated with a recumbent subretinal hyporeflective space. On the other hand, OCT findings of ONH drusen, revealsa “lumpy-bumpy” internal contour with normal adjacent RNFL thickness [19].
Along with serial photography, OCT has also show benefits monitor treatment response for patients with IIH [21]. Topographical quantitative measurements of the associated increased RNFL can be used to determine normalization of the initial elevated RNFL thickness during the resolution of papilledema [21]. Thinning of the RNFL has been documented in association of axon atrophy associated with cases of chronic papilledema [22]. Of note, a correlating study related to OCT structural optic disc morphology and visual field functional alterations have demonstrated that the severity of papilledema parallels that of its visual field sensitivity. In patients treated for IIH, the visual field defects seem to improve in parallel with documented the resolution of edema using serial OCT topographical imaging. Hence, further collaborating to the fact that OCT may be used to monitor the effectiveness of treatment in IIH.
The typical clinical picture of IIH is the presence of bilateral papilledema in a young obese female. However, it has been documented in a number of patients including, children, pregnant female’s non-obese patients, males, and even older adults, like the case presented [23-25]. This may further add to a diagnostic challenge. The diagnosis of IIH requires an associated elevated lumbar puncture of over 250mmH2O in the absence of an abnormal brain MRI and an abnormal medical examination [26]. A rare and yet, very severe atypical presentation of IIH is “Fulminant” IIH, characterize by severe and rapid vision loss within a short duration of time. Fulminant IIH is defined by acute onset of symptoms and signs associated with IIH, progressing towards severe vision loss within a short period of time. It is speculated to be related to a rapid increase in pressure within the perineural space surrounding the optic nerve can, resulting in ischemic optic neuropathy and vision loss [27-29]. It is often associated with a dramatic increase in cerebrospinal fluid opening, which surpasses the standards for classic IIH. Fulminant IIH is a medical emergency with rapid worsening of visual loss over a few days, requiring prompt aggressive management. Urgent surgery may be warranted including, temporizing measures such as repeat lumbar puncture, lumbar drainage, and IV steroids [24].
Another criterion, which is common in the clinical presentation of IIH, is obesity [30]. This is particularly important due to the growing obesity epidemic, among young females. A study concluded that the higher the BMI may be directly linked to the increased risk of severe visual loss among patient with IIH [30]. Hence, weight loss plays an important role in the management of IIH [31]. Weight loss has been shown to reduce both the signs of symptoms associated with IIH. A mere weight loss of only 5-10% of total body weight can result in improvement in both symptoms and signs [31,32].
In addition to weight loss, traditional medication therapy for IIH includes oral Acetazolamide (Diamox) [17,18,33,34]. Diamox is a Carbonic Anhydrase Inhibitor (CAI) with diuretic properties that leads to a decrease in cerebrospinal fluid. Of note, CAI’s should be used with caution in patients with sickle cell disease due to the potential to increase blood acidosis. Potential side effects of CAI include paresthesias, severe acidosis, respiratory failure and encephalopathy in patients with renal, pulmonary and hepatic disease [17].
A more recent treatment option used for IIH is Topamax (Topamirate) [35,36]. Topamax has been approved as a preventative therapy for headaches and migraines. For patients with IIH, Topamax has been found to have weak CAI properties with the added benefit as weight loss. In addition, Topamax may be suggested to help relieve the associated headaches experienced in patients with IIH. In small clinical trials, Topamax appears to have similar efficacy to Acetazolamide for treatment of mild to moderate IIH. Yet, Topamax may show further benefits due to its pharmacokinetic capabilities to cause weight loss and it’s approved used for managing migraines [31]. Of note, it is especially important for patients who are treated with Topamax to be given a comprehensive eye exam, given the acute myopia and Angle Closure Glaucoma (ACG) adverse side effects related to Topamax. The underlying mechanism of acute myopia and acute angle closure glaucoma is a ciliochoroidal effusion. This leads to ciliary body edema, which causes relaxation of zonular fibers, lens thickening, and anterior displacement of the lens-iris complex. The iris bowing forward physically blocks the drain of the eye, preventing aqueous fluid drainage, thus ultimately causing secondary ACG and myopia [31]. Management of Topamax associated acute secondary angle-closure glaucoma may be initiated, if possible, with discontinuation of the medication. In most cases, intraocular pressure decreases rapidly after the drug is stopped. Institute maximum medical therapy, including oral medications, cycloplegics&aqueous suppressants is also warranted [34]. Laser iridotomy or peripheral iridectomy is not beneficial in these cases. Topical miotics are also contraindicated in this condition; because their use may precipitate a relative pupillary block [32]. Other side effects related with Topamax use include paresthesias, drowsiness, lethargy, and decreased appetite.
Other treatment options, which has shown some promise in the treatment of IIH is bumetanide (Bumex). The specific physiological action of bumetanide is to inhibit the mechanism of glial cell volume regulation. IIH is classically associated with small ventricular size of radiographic imaging suggesting that alterations in CSF production may not be as significant as alterations in glial cell volume. This would imply that bumetanide may help in the treatment IIH by reducing glial cell volume, instead of CSF volume [37]. Bumetanide was associated with resolution of the patient’s headaches, papilledema and normalization of visual fields in a patient with IIH [35]. This case illustrates how low-dose bumetanide might be an effective way to treat patients with idiopathic intracranial hypertension by restoring the balance between cerebrospinal fluid formation and absorption and/or by altering the volume or ionic composition of the brain’s extracellular compartment [37].
Conclusion
Idiopathic Intracranial Hypertension (IIH) is a neurological condition characterized by elevated intracranial pressure in the absence of underlying conditions, resulting in bilateral optic nerve head edema. Advancement in the understanding of the condition, including associated risk factors, atypical presentations, as well as, the use of diagnostic modalities, such as OCT, has provided a better assessment of our patients with this condition. In addition, today’s treatment options are creating new opportunities for the management of IIH. Given the fact that patients with IIH presents with papilledema, primary eye care physicians, should be aware of both the condition and the most up to date information regarding this condition.
References
- Ahlskog JE, O'Neill BP. Pseudotumor cerebri. Ann Intern Med. 1982; 97: 249-256.
- Wall M, Purvin V. Idiopathic intracranial hypertension in men and the relationship to sleep apnea. Neurology. 2009; 72: 300-301.
- Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002; 59: 1492-1495.
- GiuseffiV, Wall M, Siegel PZ, Rojas PB. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumorcerebri): a case-control study. Neurology. 1991; 41: 239-244.
- Degnan AJ, Levy LM. Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011; 32: 1986-1993.
- Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain. 1991; 114: 155-180.
- Biousse V, Newman NJ, Lessell S. Audible pulsatile tinnitus in idiopathic intracranial hypertension. Neurology. 1998; 50: 1185-1186.
- Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain. 1991; 114: 155-180.
- Rowe FJ. Assessment of visual function in idiopathic intracranial hypertension. Br J Neurosurg. 2011; 25: 45-54.
- Krishna R, Kosmorsky GS, Wright KW. Pseudotumor cerebri sine papilledema with unilateral sixth nerve palsy. J Neuroophthalmol. 1998; 18: 53-55.
- Wall M. The headache profile of idiopathic intracranial hypertension. Cephalalgia. 1990; 10: 331-335.
- Friedman DI, Rausch EA. Headache diagnoses in patients with treated idiopathic intracranial hypertension. Neurology. 2002; 58: 1551-1553.
- Kahle KT, Walcott BP, Staley KJ. Resolution of headache and papilledema in idiopathic intracranial hypertension associated with inhibition of Na+-K+-2Cl- cotransport. J Child Neurol. 2011; 26: 205-208.
- Kahle KT, Walcott BP, Staley KJ. Resolution of Headache and Papilledema in Idiopathic Intracranial Hypertension Associated with Intracranial Hypertension Associated with Inhibition of Na+ K+ 2Cl- Cotransport. J Child Neurol. 2011; 26: 205-208.
- Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002; 59: 1492-1495.
- Ball AK, Clarke CE. Idiopathic intracranial hypertension. Lancet Neurol. 2006; 5: 433-442.
- Johnson LN, Krohel GB, Madsen RW, March GA. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumorcerebri). Ophthalmology. 1998; 105: 2313-2317.
- Thurtell MJ, Wall M. Idiopathic Intracranial Hypertension (PseudotumorCerebri): Recognition, Treatment, and Ongoing Management. Current Treatment Options in Neurology. 2013; 15: 1-12.
- Johnson LN, Diehl ML, Hamm CW, Sommerville DN, Petroski GF. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch Ophthalmol. 2009; 127: 45-49.
- Rebolleda G, Muñoz-Negrete FJ. Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci. 2009; 50: 5197-5200.
- Skau M, Yri H, Sander B, Gerds TA, Milea D, Jensen R. Diagnostic value of optical coherence tomography for intracranial pressure in idiopathic intracranial hypertension. Graefes Arch Clin Exp Ophthalmol. 2013; 251: 567-574.
- Salgarello T, Falsini B, Tedesco S, Galan ME, Colotto A, Scullica L. Correlation of optic nerve head tomography with visual field sensitivity in papilledema. Invest Ophthalmol Vis Sci. 2001; 42: 1487-1494.
- Rangwala LM, Liu GT. Pediatric idiopathic intracranial hypertension. Surv Ophthalmol. 2007; 52: 597-617.
- Bruce BB, Kedar S, Van Stavern GP, Corbett JJ, Newman NJ, Biousse V. Atypical idiopathic intracranial hypertension: normal BMI and older patients. Neurology. 2010; 74: 1827-1832.
- Dessardo NS, Dessardo S, Sasso A, Sarunic AV, Dezulovic MS. Pediatric idiopathic intracranial hypertension: clinical and demographic features. Coll Antropol. 2010; 34: 217-221.
- Corbett JJ, Mehta MP. Cerebrospinal fluid pressure in normal obese subjects and patients with pseudotumor cerebri. Neurology. 1983; 33: 1386-1388.
- Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007; 68: 229-232.
- Shaikh AG, Bates JH, Yeates SW, Katirji B, Devereaux MW. Fulminant idiopathic intracranial hypertension. JAMA Neurol. 2013; 70: 937-938.
- Fulminant Idiopathic Intracranial Hypertension with Malignant Systemic Hypertension–A Case Report. Neuro-Ophthalmology. 2013; 37: 120-123.
- Szewka AJ, Bruce BB, Newman NJ, Biousse V. Idiopathic intracranial hypertension: relation between obesity and visual outcomes. J Neuroophthalmol. 2013; 33: 4-8.
- Wong R, Madill SA, Pandey P, Riordan-Eva P. Idiopathic intracranial hypertension: the association between weight loss and the requirement for systemic treatment. BMC Ophthalmol. 2007; 7: 15.
- Newborg B. Pseudotumor cerebri treated by rice reduction diet. Arch Intern Med. 1974; 133: 802-807.
- Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010; 28: 593-617.
- Liu GT, Volpe NJ, Galetta SL. Pseudotumor cerebri and its medical treatment. Drugs Today (Barc). 1998; 34: 563-574.
- Celebisoy N, Gökçay F, Sirin H, Akyürekli O. Treatment of idiopathic intracranial hypertension: topiramate vs acetazolamide, an open-label study. Acta Neurol Scand. 2007; 116: 322-327.
- Fraunfelder FW, Fraunfelder FT, Keates EU. Topiramate-associated acute, bilateral, secondary angle-closure glaucoma. Ophthalmology. 2004; 111: 109-111.
- Binder DK, Horton JC, Lawton MT, McDermott MW. Idiopathic intracranial hypertension. Neurosurgery. 2004; 54: 538-551.