
Case Report
Austin J Clin Ophthalmol. 2023; 10(3): 1148.
An Unusual Case of Hyperpigmented Torpedo Maculopathy
Whyte H, Mathew C and Arora R*
Department of Ophthalmology, Salisbury District Hospital, Odstock Road, Salisbury, SP28BJ, UK
*Corresponding author: Arora RDepartment of Ophthalmology, Salisbury District Hospital, Odstock Road, Salisbury, SP28BJ, UK
Received: February 06, 2023; Accepted: March 15, 2023; Published: March 22, 2023
Abstract
Torpedo maculopathy, a solitary torpedo-shaped lesion on the macula, is a rare finding. In the literature it is classically described as being hypopigmented and located on the temporal macula. This report describes the unusual case of a hyperpigmented torpedo maculopathy located superior to the macula which is not only unreported in the literature, but may also put into question previous theories about the aetiology of torpedo maculopathy. The atypical presentation of this case highlights the importance of imaging techniques to further aid diagnosis of torpedo maculopathy.
Keywords: Torpedo maculopathy; Congenital; Macular imaging
Abbreviations: TM: Torpedo Maculopathy; RPE: Retinal Pigment Epithelium; CNV: Choroidal Neovascularization; OCT: Optical Coherence Tomography; FFA: Fundus Fluorescein Angiography; CHRPE: Congenital Hypertrophy of the Retinal Pigment Epithelium; OCTA: Optical Coherence Tomography Angiography
Introduction
First described by Roseman and Gass in 1992, Torpedo Maculopathy (TM) is a rare, congenital macular lesion involving the Retinal Pigment Epithelium (RPE) [1]. TM is classically described as a torpedo-shaped solitary, hypopigmented lesion located on the temporal macula with its tail directed towards the central macula [1-3].
This report, however, describes a case of TM with certain atypical features that have not previously been described in literature.
While most patients remain asymptomatic and stable with minimal risk of visual loss, a small cohort may develop Choroidal Neovascularization (CNV) with significant visual symptoms [4]. Hence, accurately identifying TM lesions remains important as it allows clinicians to distinguish them from progressive and visually damaging macular lesions, and triage effectively for monitoring.
Case Report
A 36-year-old caucasian male was referred from the community following an abnormal fundus exam during a routine check. He was visually asymptomatic and on examination he had normal visual acuity. Dilated fundus exam indicated a solitary, elongated, torpedo-shaped, hyperpigmented lesion with a surrounding halo in the right eye. This well demarcated lesion measured 1.25 disc diameters horizontally and 0.75 disc diameters vertically and was located superior to the macula with its apex directed towards the central macula (Figure 1). Spectral domain five-line raster of the lesion indicated a localised area of outer retinal attenuation and outer retinal and choroidal excavation with an overlying subretinal cleft. Moreover, the Optical Coherence Tomography (OCT) indicated atrophy of the outer retinal structures including a disrupted ellipsoid zone, outer nuclear and outer plexiform zones (Figure 2). The OCT of the macula indicated normal foveal contour with no macular oedema (Figure 3).
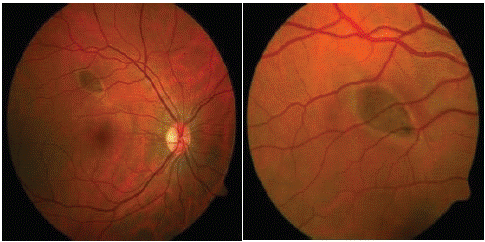
Figure 1: Colour fundus photos of the right eye indicating a well demarcated, torpedo-shaped, hyperpigmented lesion superior to the macula with the tail directed to the centre of the macula.
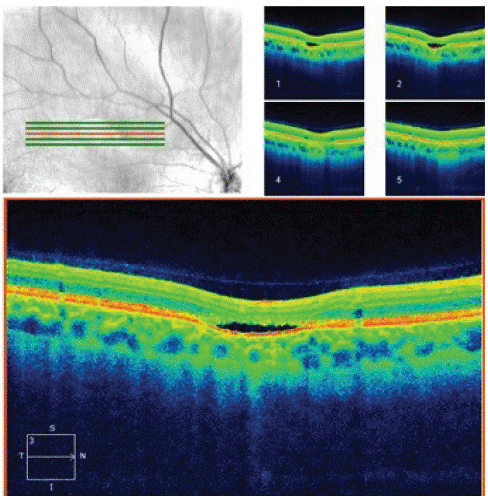
Figure 2: Spectral domain five-line raster of the lesion indicating outer retinal attenuation and excavation with overlying subretinal cleft.
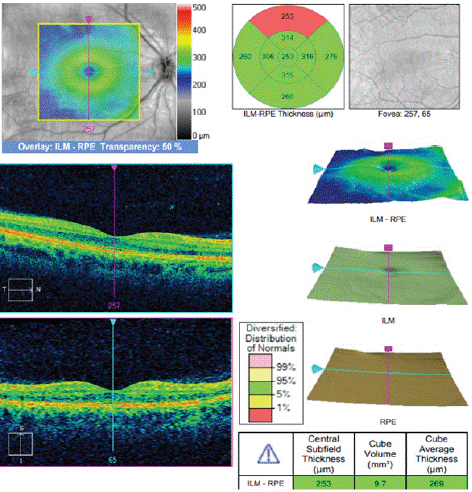
Figure 3: OCT indicates a normal macula with normal foveal contour and no fluid in the right eye.
Fundus autofluorescence indicated a hypo-autofluorescent lesion with a surrounding margin of hyper-autofluorescence which was highly suggestive of torpedo maculopathy and was further highlighted on red-free imaging (Figure 4). Moreover, Fundus Fluorescein Angiography (FFA) showed no signs of leakage.

Figure 4: Fundus Auto-Fluorescence (left image) indicates a hypo auto-fluorescent lesion and a surrounding cuff of hyper auto-fluorescence which is further highlighted on red-free (right image).
The left eye indicated a normal fundus with a normal macula (Figure 5).
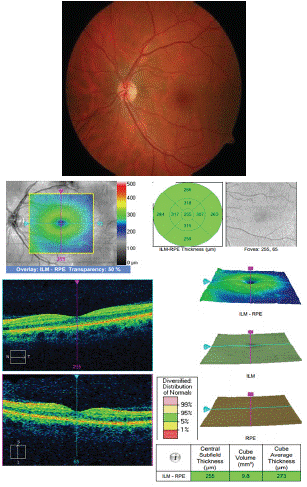
Figure 5: Colour Fundus Photo and OCT of the left eye indicating a normal fundus and normal macula.
Based on this clinical picture, supported by imaging, a diagnosis of TM was made. Given the small propensity for TM to develop CNV [4], the lesion has been monitored and has thus far remained stable with no change in vision.
Discussion
A rare entity, TM is usually asymptomatic and identified incidentally on routine fundal examination [5]. As a result, the condition may be significantly under-reported and consequently, its prevalence underestimated. The aetiology of TM remains elusive, but several theories have been proposed including developmental defects of the retinal nerve fibre layer at the level of the horizontal raphe [6], developmental defects of the RPE at the foetal temporal bulge [7] and developmental defects of the choroid and its vasculature [2]. While none of these theories have been conclusively proven, advances in imaging technology have allowed researchers to gain a finer understanding of the morphological features of these lesions, improving our understanding of the aetiology of TM and clinical diagnostic potential.
Diagnosing TM with non-classic clinical features, as in this case, requires the clinician to employ a holistic approach combining the symptomatology, clinical findings and imaging modalities.
Symptomatology
Most patients are asymptomatic with normal visual acuity as the fovea itself remains unaffected. However, perimetry and microperimetry may indicate reduced sensitivity and a scotoma corresponding to the site of the lesion. Moreover, visual function may be affected in a small subset of patients where the lesion impinges on the fovea or if the lesion progresses and produces CNV [4]. In this case, the patient was frankly asymptomatic and had perfect visual acuity. However, in the absence of microperimetry or perimetry, it is difficult to conclude if the patient had a scotoma corresponding to the area of the lesion.
Clinical Findings
Clinically, TM lesions have a hallmark appearance. It is classically described as an ellipsoid, flat, hypopigmented RPE lesion with distinct margins. They are about two and one disc diameters in size horizontally and vertically respectively. According to most cases reported in literature, TM lesions are hypopigmented and located in the temporal macula with its apex directed towards the central macula [1-3]. They are usually unilateral, although bilateral cases and satellite lesions temporal to the main lesion have been reported as well [4,8]. In this case, while the lesion shared many of these clinical characteristics, it was hyperpigmented and located superior to the macula. While there are several differential diagnoses that could be considered such as congenital toxoplasmosis, Congenital Hypertrophy of the Retinal Pigment Epithelium (CHRPE) or chorioretinal scars, multi-modal imaging helped establish a diagnosis of TM.
Diagnostic Imaging
Through the use of OCT Wong et al. classified TM lesions into two types; Type 1 lesions are characterised by attenuation of the outer retinal structures while Type 2 lesions are characterised by attenuation and excavation of the outer retinal structures with neurosensory elevation [9]. Wong et al suggested that these two distinct subtypes represented different stages in the evolution of TM lesions over several decades wherein Type 1 lesions have an average age of presentation at 17 years while type 2 lesions have an average age of presentation at 39 years [9]. On the contrary, Shirley et al. identified several cases of younger patients with type 2 lesions and therefore, suggested that the two distinct subtypes of TM lesions represented different phenotypic expressions of TM lesions rather than different stages in the evolution of TM lesions [10].
On fundus autofluorescence, TM lesions exhibit hypo-autofluorescence with a surrounding cuff of hyper-autofluorescence [11]. The hypo-autofluorescence exhibited by the lesion corresponds to atrophic RPE while the cuff of hyper-autofluorescence indicates the accumulation of lipofuscin inside retinal pigment epithelial cells [9]. These findings are corroborated on OCT which indicates attenuation of the RPE with loss of the photoreceptor layer and hyper-reflectivity of the RPE and choroid [9,12]. Additionally, type 2 lesions are also characterised by excavation of the outer RPE with a shallow subretinal cleft which is thought to represent the loss of the RPE and photoreceptors within the TM lesion [12]. This may be accompanied by thinning of the neurosensory retina [12]. In this case, the lesion exhibited hypo-autofluorescence with surrounding hyper-autofluorescence. Moreover, the OCT in this case indicated atrophy of the outer retinal structures including a disrupted ellipsoid, outer nuclear and outer plexiform zones with an overlying subretinal cleft consistent with a type 2 TM lesion.
While most patients remain asymptomatic with stable lesion appearance and are therefore discharged from follow-up, a small minority may progress to develop CNV. Indeed, a research group in Switzerland reported a case of CNV related to TM [4] while Shirley et al. reported one such case in a 15-year- old patient who had become visually symptomatic [10]. In the latter example, FFA helped identify the active CNV membrane and the patient responded well to a single dose of Ranibizumab. However, Shirley et al. concluded that the long-term prognosis in these cases remains unclear [10]. In this case, FFA showed no signs of leakage. Nevertheless, due to the remote possibility of CNV and the resulting visual consequences, patients must be counselled accordingly.
Advances in Ocular Coherence Tomography Angiography (OCTA) may not only help eliminate the need for the more invasive FFA to identify CNV associated with TM lesions, but it may also aid in understanding of its pathogenesis. Papastefanou et al. demonstrated that OCTA indicated hypo-reflectivity and reduced flow corresponding to the region of the subretinal cleft as well as an adjacent area of hyper-reflectivity corresponding to the tail of the lesion [12]. Furthermore, abnormal development of the choroid and its vasculature have been proposed as a possible cause for TM lesions [2] but further research is required to determine if this is the primary event or associated with its progression.
Conclusion
TM remains a rare and poorly understood entity and, therefore, may pose a diagnostic challenge for clinicians. However, its classic clinical appearance can be supported by imaging modalities, enabling clinicians to distinguish them from similar lesions. Indeed, in this case report, while the clinical picture was not entirely typical of TM lesions reported in literature, multi-modal imaging helped to definitively establish the diagnosis. Furthermore, given the small propensity of TM lesions to progress to CNV, it is essential that clinicians rule out CNV at presentation with FFA or OCTA. Some clinicians have advocated for annual to semi-annual monitoring with more frequent monitoring recommended for larger lesions or the presence of pigment clumping as these may render the patient more susceptible to developing CNV [13,14]. However, the patient can also monitor their vision with the Amsler grid at home. [13,14]
References
- Roseman RL, Gass JD. Solitary hypopigmented nevus of the retinal pigment epithelium in the macula. Archives of ophthalmology. 1992; 110: 1358-9.
- Teitelbaum BA, Hachey DL, Messner LV. Torpedo maculopathy. Journal of the American Optometric Association. 1997; 68: 373-6.
- Golchet PR, Jampol LM, Mathura JR, Daily MJ. Torpedo maculopathy. British journal of ophthalmology. 2010; 94: 302-6.
- Jurjevic D, Böni C, Barthelmes D, Fasler K, Becker M, et al. Torpedo maculopathy associated with choroidal neovascularization. Klinische Monatsblätter für Augenheilkunde. 2017; 234: 508-14.
- Liu Y, Moore AT. Congenital focal abnormalities of the retina and retinal pigment epithelium. Eye. 2020; 34: 1973-88.
- Williams PJ, Salek S, Prinzi RA, Bergstrom C, Hubbard III GB. Distribution patterns of torpedo maculopathy: further evidence of a congenital retinal nerve fiber layer-driven etiology. Saudi Journal of Ophthalmology. 2019; 33: 260-7.
- Shields CL, Guzman JM, Shapiro MJ, Fogel LE, Shields JA. Torpedo maculopathy at the site of the fetal “bulge”. Archives of Ophthalmology. 2010; 128: 499-501.
- Richez F, Gueudry J, Brasseur G, Muraine M. Bilateral torpedo maculopathy. Journal Francais D’ophtalmologie. 2010; 33: 296.
- Wong EN, Fraser-Bell S, Hunyor AP, Chen FK. Novel optical coherence tomography classification of torpedo maculopathy. Clinical & Experimental Ophthalmology. 2015; 43: 342-8.
- Shirley K, O’Neill M, Gamble R, Ramsey A, McLoone E. Torpedo maculopathy: disease spectrum and associated choroidal neovascularisation in a paediatric population. Eye. 2018; 32: 1315-20.
- Raval V, Rao S, Sudana P, Das T. Torpedo maculopathy. Journal of Ophthalmic & Vision Research. 2020; 15: 113-115.
- Papastefanou VP, Vázquez-Alfageme C, Keane PA, Sagoo MS. Multimodality imaging of torpedo maculopathy with swept-source, en face optical coherence tomography and optical coherence tomography angiography. Retinal Cases and Brief Reports. 2018; 12: 153-7.
- Hamm C, Shechtman D, Reynolds S. A deeper look at torpedo maculopathy. Clinical and Experimental Optometry. 2017; 100: 563-8.
- Su Y, Gurwood AS. Neurosensory retinal detachment secondary to torpedo maculopathy. Optometry-Journal of the American Optometric Association. 2010; 81: 405-7.