
Case Report
Austin J Clin Ophthalmol. 2023; 10(5): 1157.
Retinochoroidal Manifestation of Toxoplasmosis in a Young Patient
Nury Serdarov¹; Agajan Muradov¹; Galina Mamedowa¹; Ogulnur Baýrammuhammedova¹; Gozel Shirlieva¹; Mayagozel Zhutdieva¹*
¹International Center of Endocrinology and Surgery Ashgabat, Turkmenistan
²International Center of Eye Diseases Ashgabat, Turkmenistan
*Corresponding author: Mayagozel Zhutdieva Department of Consultative and Diagnostic, International center of Endocrinology and Surgery Atatürk (1972) street № 94, Ashgabat, 744028, Turkmenistan Tel: + 993 64 207526 Email: dr.zhutdieva@mail.ru
Received: April 17, 2023 Accepted: May 13, 2023 Published: May 20, 2023
Abstract
We report the case of a 15-year-old young girl, with retinochoroidal manifestation of toxoplasmosis, without vitritis. Serology for Toxoplasma gondii was positive (IgG positive /IgM negative), but PCR for Toxoplasma gondii in peripheral blood samples was negative. Blood tests including complete hemogram (total leukocyte, red blood cell and platelet count) except for hemoglobin, coagulogram (APTT, INR, and fibrin degradation product level) and routine blood biochemistry (serum urea, creatinine, and random blood sugar) were within normal limits. The serum levels of triglycerides, low density lipoproteins, high-density lipoproteins and calcium were within normal limits. Direct ophthalmoscopy of the right eye revealed a well-demarcated circular atrophic pigmented scar of about four disc diameters involving the macula. We did not initiate any therapy, because the process appeared to be active anymore. In our case demonstrate of retinochoroidal manifestation of toxoplasmosis by routine fundus examination in a young patient with uncharacteristic complaints.
Keywords: Toxoplasma gondii; Ocular toxoplasmosis; Atrophic scar
Introduction
The significance of the problem of toxoplasmic etiological manifestations is determined by their prevalence in warm-blooded mammals including humans, irreversible changes in the retina and choroid, as well as the severity and recurrent nature of the disease [1,2]. Toxoplasmosis infection is widespread throughout the world. It is caused by the obligate intracellular parasite Toxoplasma gondii for which members of the cat family are the definitive hosts. Oocysts in the cat intestine are released and spread in fecal material directly to humans and/or indirectly via the consumption of raw or partially-cooked meat prepared from other infected animals, such as pigs, sheep and chicken [3].
Ocular toxoplasmosis accounts for 15% to 17% of all intraocular inflammatory diseases. In Europe, it is the cause of more than 25% of all posterior uveitis cases [4-6]. The global prevalence of ocular toxoplasmosis strongly varies between countries and regions. For example, it is 1.8% in Japan, 8.4% in the U.S., 22.2% in Thailand and 39.8% in Colombia [7-10].
Approximately 24% patients have a blurring of vision and visual loss during acute toxoplasma retinochoroiditis involving vitreous body as a result of formation macular scar and/or optic atrophy [10-11]. Visual field loss associated with severe course of toxoplasmasmic retinochoroiditis and as a result scarring occurs close in the area of the optic disc [12]. Here we report on the clinical evolution of a retinal lesion in a young patient.
Case Presentation
We report on a case of a 15-year-old girl with decreased vision in the right eye. From the patient’s history, it is known that the patient went to the International Center of Endocrinology and Surgery Ashgabat, Turkmenistan, for difficulties with nasal breathing (nasal polyps). During a conversation with an otorhinolaryngologist, it became obvious that she also complained of poor vision in her right eye for about two years. Therefore, the patient was sent to an ophthalmologist for consultation. She was not on any local or systemic medications nor had she a family history of eye illnesses. She was not aware of any close contact with animals, about the last 3 years. However, the patient said that over the past 2 years, she sometimes consumed only lightly fried steak.
Ophthalmological Examination
On examination, visual acuity was finger counting in the right (OD) and 20/20 in the left eye (OS). Intraocular pressure was 16 mm Hg bilaterally. Slit-lamp examination revealed transparent bulbar conjunctiva and cornea, deep, clear and quiet anterior chamber with open angles, flat and intact irides. Furthermore, pupils were normal in size and shape and reactive to light, and the lens was clear. Posterior segment examination excluded vitreous haze or cells in either eye. By direct ophthalmoscopy, there was a clear view of the posterior pole in the uninfected fellow eye. The optic disc, retinal vessels, and the macula appeared normal (Figure 1b). Fundoscopic examination of the infected right eye revealed a hyperpigmented round - retinochoroidal scar of yellowish- whitish color and dark borders in the area of the macula. The size was around four diameters of the optic disc. Within the focus, there was an atrophy of the retina and the underlying uvea (Figure 1a).
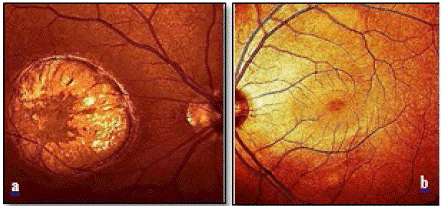
Figure 1a: Fundus photography of right eye discloses pigmented toxoplasmosis inactive scar. b: The posterior pole of the left eye with normal appearing optic disc, retinal vessels and macula.
Optical coherence tomography (OCT; Spectralis, Heidelberg Engineering, Heidelberg, Germany) in the macular area (Figure 2a) demonstrated of infective right eye typical case, active lesions are seen as yellowish-whitish foci of retinochoroiditis frequently adjacent to atrophic scar vertical size 1.087μm and horizontal size ˜ 2.086μm (Figure 2b (blue arrow)). Under the retina, the sclera is clearly visible due to atrophy of all its layers. The external limiting membrane and the outer nuclear layer and the junction zone between the outer and inner segments of photoreceptors disappear at the edges of the scar (Figure 2b).
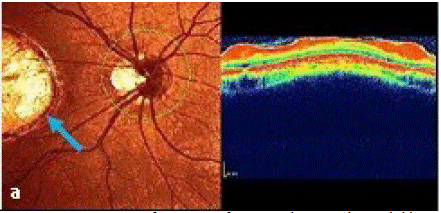
Figure 2a: Primary manifestation of a central retinochoroidal lesion in a 15-year-old girl diagnosed with ocular toxoplasmosis by clinical appearance.
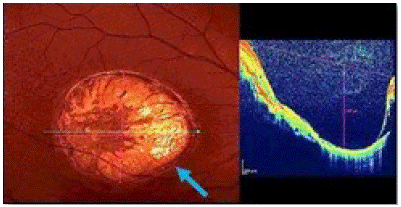
Figure 2b: Toxoplasmic retinochoroidal scar.
B-scan ultrasound biomicroscopy for both eyes to identify the absence of complications such as retinal and choroidal detachment, vitreous hemorrhages or opacities and vitreoretinal traction (Figure 3).
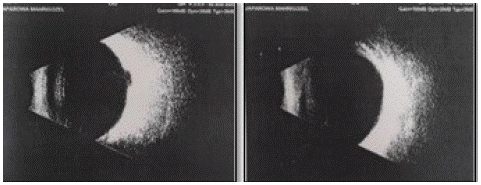
Figure 3a,b: Ultrasonography of the right and left eye.
The state of the optical fiber layer around the optic disc of both eyes is shown in Figure 4. The part of the optic disc is not changed in the left eye and are located in green area. In the right eye there is a changing in the nasal- inferior part and located in outside normal limits in the red area, superior and inferior areas in borderline
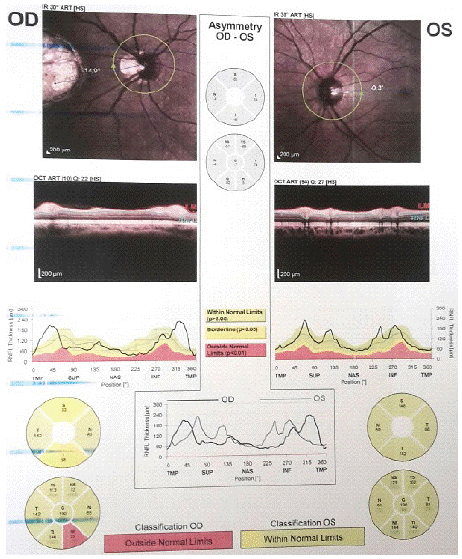
Figure 4: Bilaterally optic nerve head topography.
Laboratory Examination
The patient was hospitalized and laboratory tests were conducted, which included blood count, erythrocyte sedimentation rate, leukocytes, liver function test, antinuclear antibodies, rheumatoid factor, purified protein derivative of tuberculin (with anergy panel), Toxoplasma IgG and IgM, and Toxocara Enzyme-Linked Immunosorbent Assay (ELISA) with Toxocara Excretory-Secretory Antigen (TES-Ag). Moreover, there was no evidence for an active infection with Cytomegalovirus (CMV), Herpes Simplex Virus (HSV1 and HSV2), Varicella Zoster Virus (VZV) and rubella. Radiological chest examination was normal. Serology for specific antibody titers measured by ELISA for Toxoplasma gondii was positive for IgG and negative for IgM. PCR for T. gondii was negative.
Discussion
Toxoplasmosis is the most common parasitic intraocular infection [13-15]. Toxoplasmic retinochoroiditis typically affects the ocular posterior pole with either solitary or multiple lesions.
In humans, toxoplasmosis infection is often subclinical, presenting asymptomatic or with nonspecific symptoms and without any ocular involvement. The typical retinochoroiditis lesion is a necrotizing focal retinochoroiditis, accompanied by a vitreous reaction, and may be associated with one or more healed satellite lesions, indicative of recurrent attacks. Atypical forms of toxoplasmosis may occur in which the outer retina is involved in small punctate lesions with minimal involvement of the vitreous [16].
Most cases of retinochoroiditis are congenital. Recently, however, it has been shown that acquired infections occur more frequently than previously suspected [17]. Most of the active ocular toxoplasmosis cases represent reactivations. Clinically, congenital and acquired infections are usually asymptomatic and appear very similar.
Ocular toxoplasmosis has classically been treated includes a synergistic combination of pyrimethamine, sulfadiazine, and the use corticosteroids via intravitreal injection [18], oral [19,20] and/or intravenous [21]. Sometimes, clindamycin is added as quadruple therapy. However, there have been very few randomized trials, and there is, in fact, no consensus regarding the best treatment regimen [22]. In cases of parasitic resistance or intolerance to medical therapy, laser photocoagulation and, in cases with persistent vitreous turbidity, vitrectomy has been performed [23]. Usually, the surgical treatment is proposed only in cases with the risk of macular or optic nerve affection, with visual loss secondary to vitritis or with a high risk of retinal detachment.
Central vision may also be lost even if the macular area is not directly involved by the infection but rather develops edema secondary to a nearby lesion. The primary causes of vision loss in patients with ocular toxoplasmosis depend largely on the localization and the severity of the inflammation. Posterior pole scar, in contrast, usually cause vision loss by direct involvement of the macula or optic disc, by secondary formation of retinal folds or epiretinal membranes or, rarely, by the development of choroidal neovascularization. In most cases, it is difficult to establish the diagnosis of ocular toxoplasmosis based on clinical manifestations only, because ocular symptoms may vary, and inflammatory signs are not always present. For these reasons, in the case presented here, we want to show the diagnostic process and the morphological aspects observed with imaging methods, such as optical coherence tomography.
Conclusion
In this study, we report on a case of ocular toxoplasmosis characterized by posterior pole scar and its subsequent development into a scar in the macular region. The findings have been visualized by ophthalmoscopy and OCT. Imaging methods may be helpful to assess and track retinochoroidal and vitreous changes induced by Toxoplasma gondii, which makes these methods especially useful for the follow up.
Author Statements
Acknowledgment
The authors would like to thank Prof. Dr.med. Adrian Gericke Department of Ophthalmology, University Medical Center, Johannes Gutenberg University Mainz, Germany for proof reading the manuscript.
Competing Interests
The authors have no financial or proprietary interests related to this paper.
References
- Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: Epidemiology and course of Disease. Am J Ophthalmol. 2003; 36: 973-988.
- Subauste CS, Ajzenberg D, Kijlstra A. Review of the series “Disease of the year 2011: Toxoplasmosis” Pathophysiology of toxoplasmosis. Ocul Immunol Inflamm. 2011; 19: 297-306.
- Da Mata AP, Oréfice F. Toxoplasmosis. In: Foster CS, Vitale AT, editors. Diagnosis and Treatment of Uveitis. Philadelphia: WB Saunders Company. 2002; 385-410.
- Mc Cannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, et al. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am J Ophthalmol. 1996; 121: 35–46.
- Perkins ES, Folk J. Uveitis in London and Iowa. Ophthalmologica. 1984; 189: 36–40.
- Tran VT, Auer C, Guex-Crosier Y, Pittet N, Herbort CP. Epidemiological characteristics of uveitis in Switzerland. Int Ophthalmol. 1994; 18: 293–298.
- Keino H, Nakashima C, Watanabe T, Taki W, Hayakawa R, et al. Frequency and clinical features of intraocular inflammation in Tokyo. Clin Exp Ophthalmol. 2009; 37: 595-601.
- De-la-Torre A, López-Castillo CA, Rueda JC, Mantilla RD, Gómez-Marin JE, et al. Clinical patterns of uveitis in two ophthalmology centers in Bogota, Colombia. Clin Exp Ophthalmol. 2009; 37: 458-466.
- Sirirungsi W, Pathanapitoon K, Kongyai N, Weersink A, Leechanachai P, et al. Infectious uveitis in Thailand: Serologic analyses and clinical features. Ocul Immunol Inflamm. 2009; 17: 17-22.
- London NJ, Hovakimyan A, Cubillan, LD, Siveiro CD, Cunningham ET. Prevalence, clinical characteristics, and causes of vision loss in patients with ocular toxoplasmosis. Eur J Ophthalmol. 2011; 21: 811-819.
- Bosch-Driessen LEH, Berendschot TTJM, Ongkosuwito JV, Rothova A. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology. 2002; 109: 869–878.
- Stanford MR, Tomlin EA, Comyn O, Holland K, Pavesio C. The visual field in toxoplasmic retinochoroiditis. Br J Ophthalmol. 2005; 89: 812–814.
- Fardeau C, Romand S, Rao NA, Cassoux N, Bettembourg O, et al. Diagnosis of toxoplasmic retinochoroiditis with atypical clinical features. Am J Ophthalmol. 2002; 134: 196-203.
- Labalette P, Delhaes L, Margaron F, Fortier B, Rouland JF. Ocular toxoplasmosis after the fifth decade. Am J Ophthalmol. 2002; 133: 506-515.
- Lee M, Fong KS, Hsu LY. Optic nerve toxoplasmosis and orbital inflammation as initial presentation of AIDS. Graefe’s Arch Clin Exp Ophthalmol. 2006; 244: 1542-1544.
- Doft BH, Gass JD. Outer retinal layer toxoplasmosis. Graefe’s Arch Clin Exp Ophthalmol. 1986; 224: 78-82.
- Gilbert RE, Stanford MR. Is ocular toxoplasmosis caused by prenatal or postnatal infection? Br J Ophthalmol. 2000; 84: 224-226.
- Hazan A, Patel RM, Levinson D, Mian U, Gritz DC. A typical bilateral Toxoplasma retinochoroiditis in a bone marrow transplant patient with negative serum titers. J Ophthalmic Inflamm Infect. 2013; 3: 23.
- Yates WB, Chiong FS, Zagora, J, Post JJ, Wakefield D, et al. Ocular Toxoplasmosis in a Tertiary Referral Center in Sydney Australia-Clinical Features, Treatment, and Prognosis. Southeast Asian Pac J Ophthalmol (Phila). 2019; 8: 280-284.
- Bodaghi B, Touitou V, Fardeau C, Paris L, LeHoang P. Toxoplasmosis: new challenges for an old disease. Eye. 2012; 26: 241–244.
- Holland GN. Ocular toxoplasmosis: A global reassessment. Part II: disease manifestations and management. Am J Ophthalmol. 2004; 137: 1-17.
- Silveira C, Belfort R, Muccioli C, Holland GN, Victora CG, et al. The effect of long-term intermittent trimethoprim/ sulfamethoxazole treatment on recurrences of toxoplasmic retinochoroiditis. Am J Ophthalmol. 2002; 134: 41-46.