
Rapid Communication
Austin J Clin Ophthalmol. 2023; 10(5): 1158.
Hydrogen Peroxide-based Contact Lens Disinfection Systems Effective against SARS-CoV-2
Campolo A; Shannon P; Crary M*
Department of Microbiology, Alcon Research, LLC, Fort Worth, TX 76134, USA
*Corresponding author: Crary M Microbiology, Alcon Research LLC, 6201 South Freeway, Fort Worth, TX 76134, USA. Tel: +01-817-551-8551 Email: monica.crary@alcon.com
Received: May 17, 2023 Accepted: June 12, 2023 Published: June 19, 2023
Abstract
While SARS-CoV-2 is a largely respiratory pathogen, it has been reported that the ocular surface may present a significant opportunity for infection either via airborne droplets or by self-contamination via touching an infected surface to the eye. In particular, contaminated contact lenses present a potential vector for microorganism transmission. It is then crucial to demonstrate that contact lens disinfection solutions are effective against the SARS-CoV-2 virus. Here, we applied the International Standards Organization protocol 14729 to assess the antiviral activity of CLEAR CARE® and CLEAR CARE® PLUS, hydrogen peroxide-based contact lens disinfection systems for use with soft contact lenses. Test samples were inoculated with 5.75 log10 TCID50/mL of SARS-CoV-2 and sampled at time zero and then again after the stated disinfection time of 6 hours. Both CLEAR CARE® and CLEAR CARE® PLUS demonstrated a 4.75 log reduction of the SARS-CoV-2 virus after 6 hours. No viable virus was recovered following disinfection with CLEAR CARE® and CLEAR CARE® PLUS. This data indicates the hydrogen peroxide-based contact lens disinfection systems are extremely effective at eliminating SARS-CoV-2, and these systems may provide patients with an effective option for contact lens disinfection in the face of this pandemic.
Keywords: SARS-CoV-2; COVID-19; Ocular health; Contact lens; Hydrogen peroxide
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus that emerged in 2019 and was shortly thereafter declared a global pandemic [1]. The primary mode of transmission of SARS-CoV-2 is via infectious airborne respiratory droplets, and direct and indirect contact with nasal or oral mucosa [2]. However, the ocular surface has been reported as a potential target for SARS-CoV-2 binding and subsequent infection [3]. While reports have varied on how SARS-CoV-2 may interact with the eye, studies indicate that SARS-CoV-2 may cause ocular pathologies such as conjunctivitis and retinopathy [4]. In particular, some patients report having ocular or conjunctival symptoms before developing a fever or respiratory symptoms, virion particles have been reported in the tears [5]. Importantly, the ACE2 receptor, which SARS-CoV-2 binds to, is present in the conjunctiva, cornea, and limbus [5]. Following infection, patients demonstrate swollen endothelial cells in choroidal vessels, fibrin microthombi, and apoptotic changes of endothelial and inflammatory cells [6]. Therefore, this virus may impact contact lens wearers as the virus may be transported to the eye via contact lens where it can then bind to the ACE2 receptor, or it may promote binding by allowing aerosolized viral particles to bind to the lens, and then the eye. These potential binding opportunities presented by contaminated contact lenses would then be similar to the risk demonstrated in contact lens-mediated bacterial and Acanthamoeba keratitis cases [7].
Although few studies have reported the possibility of ocular transmission of SARS-CoV-2, there has been no clear scientific evidence of increased risk of contracting the COVID-19 disease through contact lens use. The Centers for Disease Control and Prevention reports that “hydrogen peroxide-based systems for cleaning, disinfection, and storing contact lenses should be effective against the virus that causes COVID-19” [8]. However, to our knowledge, this has yet to be demonstrated. Further, while recent studies have indicated that specific eye drops may be effective against SARS-CoV-2, these studies indicate that extremely long time periods of up to 72 hours are required to achieve only a one or two log reduction of viral load [9].
Considering the possibility of infection with SARS-CoV-2 viaocular tissue and secretions, contact lens hygiene should be followed ensuring complete disinfection between contact lens uses. Further, it is recommended that cleaning of ophthalmic lenses and instruments used in contact lens practice occur in order to minimize the transmission of SARS-CoV-2 [10]. A recent review by Bhargava et al. points towards the need for further research to determine which lens solution components will fully disinfect against this highly infectious virus [11]. Previous studies have demonstrated the antimicrobial efficacy of contemporary lens solutions containing H2O2 [12]. However, there is a paucity of data examining the antiviral activity of currently-available contact lens solutions against SARS-CoV-2. While some studies have investigated contact lens care solutions using SARS-CoV-2 surrogate viruses [13], and others have investigated contact lens care solutions with SARS-CoV-2 but only using RNA [14], there is a need to examine SARS-CoV-2 using the robust method of Vero cell plaque assay. Therefore, the objective of the present study was to evaluate the antiviral activity of hydrogen peroxide-based contact lens disinfection products against SARS-CoV-2 using an in vitro efficacy method.
Materials and Methods
All work with live SARS-CoV-2 was performed under Biosafety Level 3 containment, with a third-party contract research organization, while inactivated samples were manipulated using Biosafety Level 2 protocols.
This study used the 2019 novel coronavirus isolate hCOV-19/England/204820464/2020 (SARS-CoV-2) obtained from Biodefense and Emerging Infections Research Resources Repository (BEI Resources, Manassas, Virginia). The virus was stored at approximately 65°C until use and multiplicity of infection (MOI) was 0.01 TCID50/cell. The Vero E6 cell line, VERO C1008 (African green monkey kidney cells) from the working cell bank of BEI Resources was maintained in Dulbecco’s Minimum Essential Medium (DMEM) with 10% heat-inactivated fetal calf serum and antibiotics.
CLEAR CARE® and CLEAR CARE® PLUS (Alcon®, Fort Worth, Texas) products, which contain 3% w/v hydrogen peroxide with a platinum disc neutralizer were used in the study. CLEAR CARE® PLUS is the more recent version of CLEAR CARE® and is formulated with an additional wetting agent to enhance lens surface wettability. Test solutions were used without purification or dilution and were stored at room temperature until use.
A modified version of International Standards Organization (ISO) protocol 14729 [15] was performed to assess the antiviral activity of the inactivating agents against SARS-CoV-2. This test method followed the published standard except for ISO 14729 only specifically details five bacterial or fungal species (and this experiment was used for a viral one) and the recovery method was subsequently appropriate for the organism – please see below. Test samples of disinfection solutions were added to the paired CLEAR CARE® AOCup Lens Case and then inoculated aseptically with 5.75 log10 TCID50/mL SARS-CoV-2. Inoculum volume was maintained as 1% or less than the sample volume and the dispensed samples were thoroughly mixed immediately following inoculation to ensure complete dispersion. The lens cases were tightened immediately and stored at 20-25°C. After the stated disinfection time of 6 hours, 1mL aliquots from inoculated test formulations were collected directly from the lens cases.
Vero cell plaque assay is considered the gold standard of quantifying infectious SARS-CoV-2, as each viral particle in a well-mixed sample will create a distinct plaque, and Vero cells have repeatedly been shown to be susceptible to lytic infection by coronavirus particles due to Vero cell ACE2 expression [16,17]. All tests were performed in triplicate to ensure agreement between measurements and low standard error. Median tissue culture infectious dose (TCID50) assay was performed for the test samples, following inoculation onto confluent monolayer Vero E6 cells on 96-well plates. Vero cells in DMEM were utilized to determine the titer of viable virus after a three-day incubation.
Cytopathic effect was observed to indicate the presence or absence of virus in order to quantify the amount of surviving virus. TCID50 viral titers were calculated by the Reed and Muench method [18]. Briefly, TCID50 calculation via the Reed and Muench method is calculated by dividing the infection rate (number of cumulative positive units) by the number of cumulative positive units plus the number of cumulative negative units. Log reductions were calculated using the initial starting viral titer.
Results
CLEAR CARE® and CLEAR CARE® PLUS were inoculated with 5.75 log10 TCID50/mL SARS-CoV-2 in triplicate (Figure 1). Time zero controls were conducted to quantify the SARS-CoV-2 virus at initial inoculation. At six hours post inoculation, recovery of SARS-CoV-2 indicated no surviving virus for CLEAR CARE® and CLEAR CARE® PLUS in any replicate, demonstrated by a lack of any cytopathic effect observed in the Vero cells (Figure 1A). As such, the final log reduction for CLEAR CARE® and CLEAR CARE® PLUS is at least 4.8log, or 99.998% reduction (Figure 1B). CLEAR CARE® and CLEAR CARE® PLUS completely inactivated all viral particles by the six-hour disinfection time point, indicating a robust antiviral efficacy of the hydrogen peroxide products.
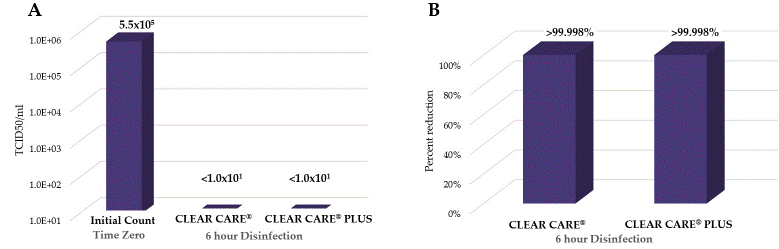
Figure 1: No viable virus was observed following disinfection with CLEAR CARE® and CLEAR CARE® PLUS. A) Quantification of surviving virus following exposure to CLEAR CARE® and CLEAR CARE® PLUS for six hours. Data is represented as mean ± SE. All three replicates from each experiment demonstrated the same amount of detectable virus, giving a SE of zero. Time zero taken from DMEM control sample. B) Percent reduction of SARS-CoV-2 after 6-hour disinfection via CLEAR CARE® and CLEAR CARE® PLUS. n=3/group.
Discussion
SARS-CoV-2, the causative agent leading to COVID-19, has produced over 600 million infections worldwide, and over 6.5 million deaths [19]. This virus is predominately a respiratory virus, but has been shown to infect hosts via the eye [5]. As contact lenses can be a transmission vector for microorganisms [20], it is critical that contact lens disinfection systems demonstrate the ability to disinfect against all potential ocular pathogens, including SARS-CoV-2.
CLEAR CARE® and CLEAR CARE® PLUS are hydrogen peroxide-based contact lens disinfecting systems available to users of soft contact lenses. These systems rely on a platinum neutralizing disc to neutralize the hydrogen peroxide for eye safety. Therefore, it is critical to test the disinfection efficacy of these systems using the real-world application of these solutions in the provided AOCup with neutralizing disc. While high percentages of hydrogen peroxide are effective against SARS-CoV-2, other in vivo low-percentage hydrogen peroxide-based products, such as mouthwash [21], have not consistently been shown to be effective against this virus. Here we show that both CLEAR CARE® and CLEAR CARE® PLUS are highly effective against SARS-CoV-2, producing a disinfection rate of at least 99.998%. This may be due to the higher 3% weight by volume which is achievable because of the platinum neutralizing disc, and also may be due to the 6-hour total disinfection time. These elements together may allow the hydrogen peroxide element of these products to disrupt the lipid layer of the virus, given then the SARS-CoV-2 virus is enveloped [22]. Further, as hydrogen peroxide is a strong oxidizing agent, these products may prevent the virus from replicating due to genomic damage [23].
While this study is the first that we are aware of involving the SARS-CoV-2 virus with hydrogen peroxide-based disinfection systems and Vero cell plaque assay, our data is in alignment with similar results reported by other groups [24]. For instance, Yasir et al. recently demonstrated that this hydrogen peroxide-based system was highly effective at disinfection against a SARS-CoV-2 viral surrogate, as it reduced the viral titer below the level of detection [13]. They further demonstrated that when a multi-purpose disinfecting solution which did not contain hydrogen peroxide or povidone iodine was used in combination with the directed rub and rinse protocol, a similarly high level of disinfection was achieved. These results have been likewise repeated by Nogueira et al., demonstrating that SARS-CoV-2 surrogates are susceptible to oxidative contact lens disinfection systems [25], and by Nogueira et al. indicating that the rub and rinse protocol in combination with phosphate buffered saline alone is effective at minimizing the viral load [24]. Thus, it is reasonable to conclude that either oxidative contact lens disinfection systems or the use of an appropriate rub and rinse protocol with a multi-purpose disinfecting solution is an acceptable method for eliminating SARS-CoV-2 from a contaminated contact lens [24]. It is important to note that the disinfection efficacy of these systems or protocols rely on patient compliance to the manufacturer directions listed on the products. Relatedly, these experiments represent in vitro results and should be reproduced in the clinical setting. Thus, an important outcome of these studies is the underscored need for the continued patient education in the ocular health community.
Conclusion
In conclusion, this study provides conclusive evidence that the CLEAR CARE® and CLEAR CARE® PLUS hydrogen peroxide-based contact lens disinfection systems are highly effective at eliminating SARS-CoV-2, as no detectable virus was seen following manufacturer directions for use.
Author Statements
Author Contributions: Conceptualization, P.S. and M.C.; methodology, M.C.; validation, M.C.; formal analysis, A.C., P.S., M.C.; investigation, M.C.; resources, P.S. and M.C.; data curation, A.C. and M.C.; writing—original draft preparation, A.C.; writing—review and editing, A.C. and M.C.; visualization, M.C.; supervision, P.S. and M.C.; project administration, P.S. and M.C.; funding acquisition, P.S. and M.C.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Alcon Research, LLC.
Conflicts of Interest
All authors are employees or contractors of Alcon Research, LLC.
References
- Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of sars-cov-2 genomes. Proc Natl Acad Sci USA. 2020; 117: 9241-9243.
- Chen Z, Yuan G, Duan F, Wu K. Ocular involvement in coronavirus disease 2019: Up-to-date information on its manifestation, testing, transmission, and prevention. Frontiers in medicine. 2020; 7: 569126.
- Sun CB, Wang YY, Liu GH, Liu Z. Role of the eye in transmitting human coronavirus: What we know and what we do not know. Frontiers in public health. 2020; 8: 155.
- Lourenco Nogueira C, Boegel SJ, Shukla M, Ngo W, et al. Antiviral activity of contemporary contact lens care solutions against two human seasonal coronavirus strains. Pathogens. 2022; 11: 472.
- Dawood AA. Transmission of sars cov-2 virus through the ocular mucosa worth taking precautions. Vacunas (English Edition). 2021; 22: 56-57.
- Reinhold A, Tzankov A, Matter MS, Mihic-Probst D, Scholl HPN, et al. Ocular pathology and occasionally detectable intraocular severe acute respiratory syndrome coronavirus-2 rna in five fatal coronavirus disease-19 cases. Ophthalmic research. 2021; 64: 785-792.
- Eltis M. Contact-lens-related microbial keratitis: Case report and review. Journal of Optometry. 2011; 4: 122-127.
- Contact Lens Care Systems & Solutions. Available Online: https://www.cdc.gov/contactlenses/care-systems.html (Accessed on 13 October 2021).
- B+L: Investigational In Vitro Data Indicates Complete Inactivation of SARS-CoV-2 with Lumify and Besivance Eye Drops. Available online at https://eyewire.news/news/bausch-health-announces-investigational-in-vitro-data-indicating-complete-inactivation-of-sars-cov-2-with-lumify-and-besivance-eye-drops (Accessed 13 October 2021).
- Zeri F, Naroo SA. Contact lens practice in the time of covid-19. Cont Lens Anterior Eye. 2020; 43: 193-195.
- Bhargava R. Contact lens use at the time of sars-cov-2 pandemic for healthcare workers. The Indian journal of medical research. 2020; 151: 392-394.
- Gabriel MM, McAnally C, Chen H, Srinivasan S, Manoj V, et al. Hydrogen peroxide disinfecting solution for gas permeable contact lenses: A review of the antimicrobial efficacy, compatibility, and safety performance of a one-step lens care system. Clin Optom (Auckl). 2021; 13: 7-14.
- Yasir M, Kumar Vijay A, Willcox M. Antiviral effect of multipurpose contact lens disinfecting solutions against coronavirus. Contact lens & anterior eye: the journal of the British Contact Lens Association. 2021; 45: 101513-101513.
- Veugen JMJ, Nuijts RMMA, van den Biggelaar FJHM, Gijs M, Savelkoul PHM, et al. Effectiveness of commonly used contact lens disinfectants against sars-cov-2. Eye & Contact Lens. 2022; 48: 362-368.
- ISO 14729:2001/A1:2010. Ophthalmic Optics–Contact Lens Care Products–Microbiological Requirements and Test Methods for Products and Regimens for Hygienic Management of Contact Lenses. Geneva, Switzerland: International Organization for Standardization, 2010.
- Mendoza EJ, Manguiat K, Wood H, Drebot M. Two detailed plaque assay protocols for the quantification of infectious sars-cov-2. Current protocols in microbiology. 2020; 57: ecpmc105.
- Mossel EC, Huang C, Narayanan K, Makino S, Tesh RB, et al. Exogenous ace2 expression allows refractory cell lines to support severe acute respiratory syndrome coronavirus replication. Journal of virology. 2005; 79: 3846-3850.
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. American Journal of Epidemiology. 1938; 27: 493-497.
- World Health Organization Coronavirus Dashboard. Available Online: https://covid19.who.int/ (Accessed 12 October 2022).
- Szczotka-Flynn LB, Imamura Y, Chandra J, Yu C, Mukherjee PK, et al. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea. 2009; 28: 918-926.
- Ather A, Parolia A, Ruparel NB. Efficacy of mouth rinses against sars-cov-2: A scoping review. Frontiers in Dental Medicine. 2021, 2.
- Eslami H, Das S, Zhou T, Müller-Plathe F. How alcoholic disinfectants affect coronavirus model membranes: Membrane fluidity, permeability, and disintegration. The journal of physical chemistry. B 2020; 124: 10374-10385.
- Termini J. Hydroperoxide-induced DNA damage and mutations. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2000; 450: 107-124.
- Nogueira CL, Boegel SJ, Shukla M, Ngo W, Jones L, et al. The impact of a rub and rinse regimen on removal of human coronaviruses from contemporary contact lens materials. Contact Lens and Anterior Eye. 2022; 45: 101719.
- Nogueira C, Boegel S, Pharm M, Ngo W, Jones L, et al. Antiviral Activity of Contemporary Contact Lens Solutions Against Human Seasonal Coronavirus Strains. American Academy of Optometry Scientific Session. 2021.