
Special Article - Diabetic Retinopathy: Clinical Cases and Images
Austin J Clin Ophthalmol. 2015; 2(4): 1056.
The Effect of Topical Anesthesia on Pharmacological Mydriasis in Diabetic Patients Depends on the Presence of Retinopathy
Clausen S, Jørgensen CM and Bek T*
Department of Ophthalmology, Aarhus University Hospital, Denmark
*Corresponding author: Bek T, Department of Ophthalmology, Aarhus University Hospital, DK-8000 Aarhus C, Denmark
Received: July 23, 2015; Accepted: September 10, 2015; Published: September 21, 2015
Abstract
Purpose: Instillation of topical anesthetics with or without preservatives in normal persons has been shown to enhance the effect of mydriatic eye drops. The purpose of the present investigation was to study whether a similar effect can be observed in diabetic patients screened for retinopathy and whether the effect depends on the presence of retinopathy.
Methods: Thirty-six patients attending a screening programme for diabetic retinopathy were randomized to receive local anesthetic eye drops on one eye, followed by instillation of both a sympathomimetic and a parasympatholytic eye drop in both eyes, which was repeated after 20 minutes. The pupil diameter in both eyes was measured with 5 minutes intervals until 35 minutes after the first application of mydriatics, and the responses in the two eyes were compared.
Results: The pupil diameter increased significantly throughout the observarion period in both eyes (p<0.0001), and after 10 minutes the effect was significantly higher in the patients who had received topical anesthesia. The increase in the pupil diameter in this group was significantly higher from 5-10 minutes after the first (p<0.003) and from 0-5 minutes after the second (p<0.01) application of mydriatic eye drops. The patients with retinopathy had significantly (p<0.05) smaller baseline pupil diameter than the patients with no retinopathy, and this difference persisted throughout the experiment.
Conclusions: Topical anesthesia enhances pharmacological mydriasis in patients during screening for diabetic retinopathy, but pupil size before and after the intervention depends on the presence of retinopathy.
Keywords: Topical anesthesia; Mydriasis; Screening; Diabetic retinopathy
Introduction
Screening for diabetic retinopathy requires pharmacological mydriasis for the retina to be studied in detail and documented by fundus photography [1]. The mydriatic effect can be achieved by topical application of parasympatholytic and/or sympatomimetic drugs with the side effect of inducing a short-lived smarting or burning sensation. In normal persons it has been shown that instillation of topical anesthetics with or without preservatives prior to the mydriatic eye drops can reduce the irritation induced by the mydriatic eye drops [2-4], and may increase corneal permeability to allow a faster onset and a more pronounced mydriatic effect [5-7].
It is likely that the influence of topical anesthesia on the effect of mydriatic compounds may be different in diabetic patients because pupil motility and corneal sensitivity are reduced due to autonomic neuropathy [2,8], and because the effect of topical anesthesia on corneal epithelial barrier permeability in diabetic patients differs from that of normal persons [9]. However, the effect of topical anesthesia on pharmacological mydriasis has not been studied in diabetic patients.
Therefore, 36 non-selected patients attending a screening programme for diabetic retinopathy were randomized for local anesthetic eye drops on one eye, followed by the routinely used instillation of both a sympathomimetic and a parasympatholytic compound on both eyes which was repeated after 20 minutes. The pupil diameter in both eyes was measured with 5 minutes intervals until 35 minutes after the first application of mydriatics, and the response was compared between the intervention eye and the control eye.
Materials and Methods
Design
Prospective randomized interventional study of the mydriatic effect of metaoxedrin and phenylephrin preceded by topical application of oxybuprocain with benzalkonium chloride preservative in one eye and in patients participating in a screening programme for diabetic retinopathy.
Patients
Thirty-six patients (twenty men and sixteen women) scheduled for examination in the screening clinic for diabetic retinopathy at the Department of Ophthalmology, Aarhus University Hospital, were studied. This clinic performs approximately 4,000 screening examinations for diabetic retinopathy annually, which corresponds to approximately twenty examinations each working day with no preferences for booking of particular patient types on specific times of the day. The patients were recruited prospectively among the two first patients scheduled in the programme on 2-3 days each week over a period of six months. Exclusion criteria were: Pregnant and lactating women and patients with any known previous or present ocular disease apart from diabetic retinopathy.
Written information about the study was sent by ordinary mail to the patients at least three weeks before the examination and included an e-mail address and a phone number to indicate an interest for participation in the study. One hundred and fourteen patients from 77 examination days were considered for an invitation of which six patients were excluded due to systemic conditions (2), previous ocular surgery (3) or acute conjunctivitis (1). Among the remaining 108 patients 38 reported that they were willing to participate. At the day of the examination one patient was excluded because of reminding of previous operation for cataract and another patient because of accidental deviation from the examination protocol, which resulted in the inclusion of 36 patients. These patients consisted of twelve patients with type 1 diabetes mellitus defined as onset of diabetes before the age of 40 and a need for treatment with insulin within one year after the diagnosis, and 24 patients defined as having type 2 diabetes mellitus. The iris colour was blue in 34 patients and brown in two patients. Twenty of the patients had no retinopathy, whereas the remaining sixteen patients had retinopathy.
A preliminary power analysis had shown that with an accuracy of 0.2 mm in the measurement of the pupil diameter, 31 patients would have to be included in order to detect a difference between the two eyes, assuming a power of 0.8 and p<0.05.
The study was approved by the Regional Committee for Scientific Ethics, The Danish Medicines Agency, The Danish Data Protection Agency and was monitored by the GCP unit at Aarhus University Hospital.
Ophthalmological examination
The patients who were interested in participating were further informed about the practical procedures, followed by the signing of a written consent. Subsequently, the patient was asked about the time of onset of diabetes mellitus, previous and current general and ophthalmological diseases, possible visual problems and current treatment of diabetes mellitus, and the patient record was updated if relevant. Finally, best corrected visual acuity was measured using ETDRS visual acuity charts. The diameter of the pupil was measured using an autorefractor (Nidek Tonoref II, Maehama, Hiroishicho, Gamagori, Aichi, Japan). Measurements of refraction by this apparatus includes photography of the iris plane under mesopic conditions in order to measure the pupil diameter to estimate the size of the optical zone. The image of the pupil is shown on a computer screen, and two vertical bars are moved manually until they align with respectively the nasal and the temporal edges of the pupil. When the examiner presses a button the horizontal distance between the two bars is displayed on the screen with a precision of 0.1 mm. This distance and the precision has been shown to be comparable with that obtained with Colvard’s electrical pupillometer [10].
Eye drops
Oxybuprocain 0.8 mg/ml containing benzalkonium chloride 0.01 mg/ml, borate acide 1.59 mg/ml and disodium EDTA 0.05 mg/ml, purchased from the hospital pharmacy at Aarhus University Hospital. Metaoxedrin 10 % containing benzalkonium chloride 0.1 mg/ml and disodium EDTA 0.5 mg/ml was purchased from the hospital pharmacy, Skanderborg, Denmark. Mydriacyl 1 % containing benzalkoniumchloride 0.1mg/ml (50% solution), disodium EDTA 0.1 mg/ml and sodium chloride 7.0 mg/ml was purchased from Alcon, Puurs, Belgium.
Procedure
The experimental protocol is shown in Figure 1. The right eye was selected as the test eye and the left eye as the control eye in the first included patient, which was subsequently alternated for each successively included patient. The instillation of mydriatic eye drops and the measurement of pupil diameter were in all patients performed initially in the right eye, immediately followed by instillation in the left eye. The sequence of events in the examination was as follows:
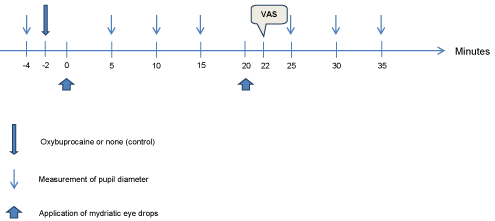
Figure 1: The experimental protocol. The time where patient discomfort was
evaluated on a visual analogue scale is indicated by VAS.
1. The pupil diameter in both eyes was measured at baseline, followed by a resting period lasting up to 2 minutes.
2. Oxybuprocain was administered to the intervention eye, followed by a resting period lasting between 1 and 2 minutes.
3. Metaoxedrin was administered to both eyes, followed by 30 seconds of rest, administration of mydriacyl to both eyes.
4. Measurement of the pupil diameter was performed 5, 10 and 15 minutes after the first administration of eye drops in step 3.
5. Subsequently, the patient rested 5 minutes and the procedures in steps 3 and 4 were repeated.
6. Two minutes after the last administration of eye drops the patient was asked to rate the degree of discomfort by the preceding administrations of eye drops using a visual analogue scale (VAS) ranging from 0 = no discomfort to 10 = maximal discomfort.
After dilatation of the pupil the routine examination was finished by measurement of the blood pressure using an oscillometric technique (Omron Healthcare B.V, Hoofddorp, The Netherlands), and the height and weight (SECA, Hamburg, Germany). Finally, fundus photography was performed on both eyes using a Canon CF 60Z fundus camera (Canon, Tokyo, Japan) with one photograph centered on the fovea and a nasally displaced photograph centered on the optic nerve head.
Data analysis
For both the intervention and the control eye the change in the effect over time were tested using repeated measures one-way ANOVA. Student’s paired t-test was used to test the difference in pupil size and the patient’s indication of the degree of discomfort after topical application of mydriatics between the control eye and the intervention eye, and to test whether the inter-eye difference after each intervention subtracted by the inter-eye difference from the immediately preceding measurement deviated significantly from zero.
Results
The measurements of pupil diameters are shown in (Table 1). It appears that the pupil diameter increased significantly throughout the observation period in both the intervention eye and the control eye (repeated measures one-way ANOVA, p<0.0001 for both comparisons). Furthermore, it appears that at all measurements after 10 minutes, the pupil diameter was larger in the intervention group than in the control group.
Time from start
0 min
5 min
10 min
15 min
25 min
30 min
35 min
Intervention eye (mm)
5.57 + 0.16
5.81 ± 0.15
6.58 ± 0.15
7.02 ± 0.14
7.59 ± 0.15
7.79 ± 0.15
7.98 ± 0.15
Control eye (mm)
5.61 + 0.16
5,76 ± 0.15
6.36 ± 0.14
6.80 ± 0.14
7.26 ± 0.14
7.45 ± 0.14
7.61 ± 0.14
p
0.51
0.60
0.002
0.0001
< 0.0001
< 0.0001
< 0.0001
Table 1: The pupil diameter (mean±SEM) of the two intervention and the control eye over time.
The changes in the inter-eye difference in pupil diameter between each successive examination are shown in (Table 2). It appears that the increase in the pupil diameter was significantly higher in the intervention group from 5-10 minutes after the first application of mydriatic eye drops (p<0.003) and from 0-5 minutes after the second application of mydriatic eye drops (p<0.01).
First application
Second application
Time from application
0-5 min
5-10 min
10-15 min
0-5 min
5-10 min
10-15 min
Difference
(mm)
0.09 ± 0.05
0.17 ± 0.05
0.00 ± 0.04
0.11 ± 0.04
0.02 ± 0.02
0.03 ± 0.02
p
0.09
0.003*
0.94
0.01*
0.34
0.13
Table 2: The changes in the inter-eye difference in pupil diameter between each measurement (mean±SEM).
At the baseline examination (mean±SEM) the patients with retinopathy had significantly (p<0.05) smaller pupil diameter (5.33±0.17mm) than the patients with no retinopathy (5.77±0.14mm). The difference was still present after 35 minutes where the pupil diameter was 7.59±0.16mm in patients with retinopathy and 7.94±0.13mm in patients with no retinopathy. Accordingly, there was no significant difference in the changes between diameter measurements among the two groups over time (p>0.08 for all comparisons).
The VAS score (mean±SEM) obtained after the second administration of mydriatic eye drops was significantly lower (p <0.001) in the intervention group (1.36±0.03) than in the control group (4.14±0.05).
Discussion
The present study is the first to evalute the influence of topical anesthesia on the effect of mydratic eye drops in diabetic patients. The study was undertaken in order to provide more firm evidence for the effect of routines used in screening programmes for diabetic retinopathy [11-12] and used standardized illumination, trained personnel and equipment that provided reproducible measurements in order to ensure that the measurements of pupil diameter were accurate [10]. The duration of the period for observing pupil size after application of eye drops was set to 35 minutes, since this was considered to be a maximum acceptable waiting time for patients in our routine screening setting. However, since the pupil diameter had not reached a stable diameter at that time it cannot be excluded that effects of the interventions after this period may have been overlooked.
Previous studies of the influence of topical anesthesia on pharmacological mydriasis have been conflicting with some studies showing that this additional procedure may result in a larger pupil size [6,7,13], whereas other studies have not been able to show this effect [4,14]. The findings of the present study with an accelerated effect at specific times after the intervention suggest that this discrepeancy might be due to differences in study design with measurements of changes in pupil size having been performed at different times after instillation of the mydriatic eye drops. Although the study confirmed that topical anesthesia can accelerate pharmacological mydriasis [5], the design was not suitable for studying the influence of eye pigmentation and support evidence that this factor may prolong the mydriatic effect after topical anesthesia [15]. Although the observation period was too short for the pupil diameter to reach a stable level, it is likely the effect of topical anesthesia on the rate of dilatation had had less influence on the final magnitude of the dilatation [4,14] suggesting that the effect of topical anesthesia is due to an increase in bioavailabilty and not to a sensitization of the mydriatic eye drops.
The observed response with accelerated mydriasis is consistent with findings of increased corneal permeability after application of topical anesthesia [3], after application of the preservatives benzalkonium chloride [16,17] or ethylen di-amine tetra acetate (EDTA) combined with boric acid [18]. These compounds were all constituents of the employed anesthetic eye drops. In addition to the increased corneal permeability induced by the preservatives, the local anesthetics may also influence the removal of eye drops because of reduced tear production when the mydriatic eye drops induce less irritation. However, in order to optimize the effect of local anesthetic eye drops, the effect on mydriasis of each of the active components should be studied separately.
The study protocol implied two applications of mydriatic eye drops [19], and the fact that a larger change in pupil size was obtained in the intervention eye 10 minutes after the first application, but 5 minutes after the second application of mydriatic eye drops which highlights the complex pharmacological effects induced by topical eye drops before applying mydriatic eye drops.
At baseline the studied diabetic patients with retinopathy had a smaller pupil size than patients without retinopathy, probably due to autonomic neuropathy [2,8]. However, the absolute increase in the pupil diameter induced by the mydriatic eye drops did not differ among the two patient groups which highlight the challenge in reaching a sufficient pupil size for photography in patients with more severe retinopathy where the results of the screening examination may be crucial for planning the strategy for further control and treatment [20]. Additionally, topical anesthesia significantly reduced the subjective irritation induced by mydriatic eye drops similarly to what has been found in normal persons [21], and indicates that the irritating effect of eye drops is not necessarily reduced in diabetic patients secondary to autonomic neuropathy.
In conclusion, the study has shown the usefulness of topical anesthesia for enhancing pharmacological mydriasis before examination of the ocular fundus during screening for diabetic retinopathy, but also shows that the intervention may not have sufficient effect in patients with diabetic retinopathy. Local anesthesia reduces the subjective irritation of mydriatic eye drops, but may induce allergic reactions and increase the risk of corneal abrasio and other damage due to reduced sensitivity in the ocular surfaces. Therefore, the need for local anesthesia for pharmacological mydriasis depends on a balancing of these advantages and disadvantages. The study points to a need for a future exploring of the relative contribution of anesthetics and preservatives and the pharmacokinetics of these compounds for modifying the effect of mydriatic eye drops.
Acknowledgement
The nurses in the screening clinic for diabetic retinopathy at Aarhus University Hospital, are gratefully acknowledged.
References
- Stefánsson E, Bek T, Porta M, Larsen N, Kristinsson JK, Agardh E. Screening and prevention of diabetic blindness. Acta Ophthalmol Scand. 2000; 78: 374-385.
- Touzeau O, Levet L, Borderie V, Bouchard P, Laroche L. Anterior segment of the eye and diabetes mellitus. J Fr Ophtalmol. 2004; 27: 859-870.
- Behndig A. Effects on pupil size and accommodation from topical lidocaine hydrochloride and tetracaine hydrochloride. J Ocul Pharmacol Ther. 2007; 23: 591-598.
- Haddad DE, Rosenfield M, Portello JK, Krumholz DM. Does prior instillation of a topical anaesthetic alter the pupillary mydriasis produced by tropicamide (0.5%)? Ophthalmic Physiol Opt. 2007; 27: 311-314.
- Siu AW, Sum AC, Lee DT, Tam KW, Chan SW. Prior topical anesthesia reduces time to full cycloplegia in Chinese. Jpn J Ophthalmol. 1999; 43: 466-471.
- Ghose S, Garodia VK, Sachdev MS, Kumar H, Biswas NR, Pandey RM. Evaluation of potentiating effect of a drop of lignocaine on tropicamide-induced mydriasis. Invest Ophthalmol Vis Sci. 2001; 42: 1581-1585.
- Claesson M, Johansson M, Behndig A. Mydriasis with different preparations of topically administered lidocaine hydrochloride. J Cataract Refract Surg. 2009; 35: 277-281.
- Pittasch D, Lobmann R, Behrens-Baumann W, Lehnert H. Pupil signs of sympathetic autonomic neuropathy in patients with type 1 diabetes. Diabetes Care. 2002; 25: 1545-1550.
- Stolwijk TR, van Best JA, Boor JP, Lemkes HH, Oosterhuis JA. Corneal epithelial barrier function after oxybuprocaine provocation in diabetics. Invest Ophthalmol Vis Sci. 1990; 31: 436-439.
- Mantry S, Banerjee S, Naroo S, Shah S. Scotopic measurement of normal pupil size with the Colvard pupillometer and the Nidek auto-refractor. Cont Lens Anterior Eye. 2005; 28: 53-56.
- Bek T, Lund-Andersen H, Hansen AB, Johnsen KB, Sandbaek A, Lauritzen T. The prevalence of diabetic retinopathy in patients with screen-detected type 2 diabetes in Denmark: the ADDITION study. Acta Ophthalmol. 2009; 87: 270-274.
- Ghanchi F. Diabetic Retinopathy Guidelines Working Group. The Royal College of Ophthalmologists' clinical guidelines for diabetic retinopathy: a summary. Eye (Lond). 2013; 27: 285-287.
- Lyle WM, Bobier WR. Effects of topical anesthetics on phenylephrine-induced mydriasis. Am J Optom Physiol Opt. 1977; 54: 276-281.
- Siderov J, Chuang SM, Ian K, Prassinos G, Tziortzi E, Wong JY. Effect of proparacaine on tropicamide-induced mydriasis. Optom Vis Sci. 1997; 74: 1039-1043.
- Mordi JA, Lyle WM, Mousa GY. Does prior instillation of a topical anesthetic enhance the effect of tropicamide? Am J Optom Physiol Opt. 1986; 63: 290-293.
- Burstein NL. Preservative cytotoxic threshold for benzalkonium chloride and chlorhexidine digluconate in cat and rabbit corneas. Invest Ophthalmol Vis Sci. 1980; 19: 308-313.
- McCarey B, Edelhauser H. In vivo corneal epithelial permeability following treatment with prostaglandin analoges with or without benzalkonium chloride. J Ocular Pharmacol Ther. 2007; 23: 445-451.
- Kikuchi T, Suzuki M, Kusai A, Iseki K, Sasaki H. Synergistic effect of EDTA and boric acid on corneal penetration of CS-088. Int J Pharma. 2005; 290: 83-89.
- Jethani J, Solanki H, Nayak A. Effect of the single-drop mydriatic combination of 0.8% tropicamide with 5% phenylephrine with multiple applications of the same drop: a randomized controlled trial. Indian J Ophthalmol. 2011; 59: 323-325.
- Jeppesen P, Bek T. The occurrence and causes of registered blindness in diabetes patients in Arhus County, Denmark. Acta Ophthalmol Scand. 2004; 82: 526-530.
- Seabaugh VM, Chambers WA, Green S, Gupta KC, Hill RN, Hurley PM, et al. Use of ophthalmic topical anaesthetics. Food Chem Toxicol. 1993; 31: 95-98.