
Research Article
Austin J Clin Ophthalmol. 2018; 5(1): 1088.
Visual Acuity Following Transscleral Cyclophotocoagulation as Primary Surgical Treatment for Primary Open Angle Glaucoma
Lei-Ai Lim¹*, Inderraj Hanspal² and Daniel Byles³
¹Moorfields Eye Hospital Foundation NHS Trust,UK
²West Suffolk NHS Trust,UK
³Royal Devon and Exeter Foundation NHS Trust, UK
*Corresponding author: Lei-Ai Lim, Taunton and Somerset Foundation NHS Trust, Musgrove Park Hospital, Taunton, Somerset TA1 5DA, UK
Received: February 13, 2018; Accepted: March 12, 2018; Published: March 22, 2018
Abstract
Purpose: The aim of this study was to report the effect of TSCP as primary surgical treatment for primary open angle glaucoma (POAG) on eyes with good pre-treatment visual acuity.
Methods: Retrospective case notes review of patients who underwent TSCP for POAG between January 2002 and December 2006 at the West of England Eye Unit. Only patients with a pre-treatment Snellen visual acuity of 6/12 or better were included. 1 year follow up data was analysed.
Results: 27 of 43 patients underwent TSCP for POAG from January 2002 and December 2006 had visual acuity of 6/12 or better prior to TSCP. 15/27 (56%) maintained their visual acuity within 1 line at 1 year. 12/27 (44%) lost more than one Snellen line of acuity. The mean pre-treatment IOP was 22mmHg (SE 1.43). Following treatment the IOP at 1, 6 and 12 months were: 14mmHg (SE 1.49), 13mmHg (SE 0.94) and 13mmHg (SE 0.90) respectively (P<0.001 one-way repeated measures ANOVA).
Comparing the group maintaining vision with the group losing vision, there was no significant difference between the pre-treatment IOP (0.37 unpaired t-test) or the IOP reduction (p=0.18 unpaired t-test). The pre-treatment visual fields showed mean deviation values of -12.93 (SE 1.82) in the group that maintained visual acuity and -19.17 (SE 1.96) in the group that lost visual acuity (P=0.018 unpaired t-test).
Conclusions: TSCP for patients with POAG and good pre-treatment acuities carries a significant risk of visual deterioration, which can sometimes be severe. TSCP should be used with caution in this group of patients with advanced visual field loss.
Keywords: Primary Open Angle Glaucoma; Transscleral cyclophotocoagulation; Pretreatment
Introduction
Transscleral cyclophotocoagulation (TSCP) has an established role in the management of refractory glaucoma [1-9]. There are fewer publications with regards to its use as primary surgical treatment for primary open angle glaucoma (POAG) in patients with good pretreatment visual acuity [10-13]. In particular, the effect of TSCP on visual acuity in patients with POAG and good pre-treatment acuity is poorly documented in the literature. This study focussed on the effect of TSCP on patients with good pretreatment visual acuity for medically uncontrolled POAG.
Methods
A retrospective case note review was conducted of all patients who underwent TSCP for POAG between January 2002 and December 2006 at the West of England Eye Unit. All patients were treated with TSCP by a single surgeon, using the same treatment protocol. Data collected included age, sex, Snellen visual acuity and intraocular pressure (IOP measured by Goldman applanation tonometry) pre- treatment, at 1 month, 6 months and 12 months following TSCP. The number of anti-glaucoma medications pre and post TSCP and pre-treatment mean deviation as measured by the Humphrey field analyser using the SITA 24-2 protocol were also recorded.
Only patients with pre-treatment best corrected Snellen acuity of 6/12 or better were included in the final analysis.
The indication of TSCP was IOP uncontrolled with maximum tolerated medical treatment, unsuitability (either due to age or complex medical conditions) or refusal of filtration surgery.
All TSCP were performed following subtenon’s local anaesthesia as day case procedures using the OcuLight Slx semiconductor diode 810nm laser with disposable contact G probe. All patients had informed consent following discussion of treatment options and explanation of the procedure. Transillumination was performed to determine the anterior segment morphology prior to delivery in each case of 32 shots (8 per quadrant over 360 degrees) with default energy setting of 3W and 1.5sec. If a “pop” sound was audible during treatment, the power was decreased by 200mW until no further “pops” was heard. All patients received topical Betnesol ointment immediately following treatment and were instructed to instil Guttae Maxitrol for 4-6 weeks post treatment along with pre-treatment antiglaucoma medication. None of the patients were on oral Acetazolamide prior to TSCP. Retreatment settings were unchanged.
All findings were reported as means with standard error (SE) of the mean. All p values were calculated using unpaired two-way t-test unless stated otherwise.
Results
A total of 43 patients with POAG had cyclodiode laser between January 2002 and December 2006. 27 patients with pre-treatment Snellen visual acuity of 6/12 or better were included in the final analysis. The 16 patients who were excluded all had visual acuities of 6/60 or worse.
The mean age was 81 years. There were 13 (48%) male and 14 (52%) females.
At one year follow up, 15 of the 27 (56%) patients maintained their Snellen visual acuity within 1 line of their pretreatment visual acuity. 12 out of the 27 patients (44%) had reduction of visual acuity of greater than one line on the Snellen chart. Of the latter, 7 patients (26%) lost 2 lines, 3 patients (11%) lost 3 lines and 2 patients (7%) went from 6/6 to 2/60 and HM respectively (Figure1). The reason for loss of vision was progression of glaucomatous optic neuropathy (GON).
The mean pre-treatment IOP was 22mmHg (SE 4.76). The mean post treatment IOPs were 14mmHg (SE 7.69) at 1 month, 13mmHg (SE 4.79) at 6 months and 13mmHg (SE 3.94) at 1 year. The mean reduction in IOP with TSCP did not differ in the group that maintained visual acuity compared to the group that lost vision. In the group that maintained visual acuity, the mean pre-treatment IOP was 22mmHg (SE 5.16) and the mean post-treatment IOPs were 14mmHg (SE 5.46) at 1 month, 13mmHg (SE 3.04) at 6 months and 14mmHg (SE 3.71) at 1 year. In the group with significant reduction in visual acuity, the mean pre-treatment IOP was 21mmHg (SE 4.25) and the mean post-treatment IOPs were 14mmHg (SE 10.09) at 1 month, 12mmHg (SE 6.63) at 6 months and 12mmHg (SE 3.94) at 1 year (Figure 2). The percentage IOP reduction for the group that maintained visual acuity was 31.8% and that for the group that lost visual acuity was 42.1%.
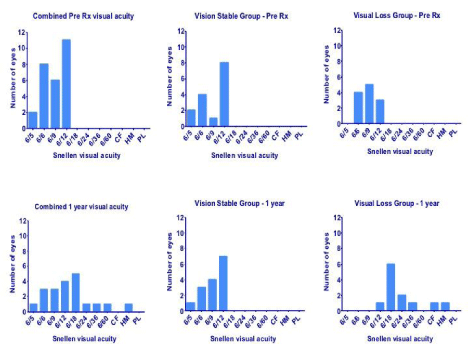
Figure 1:
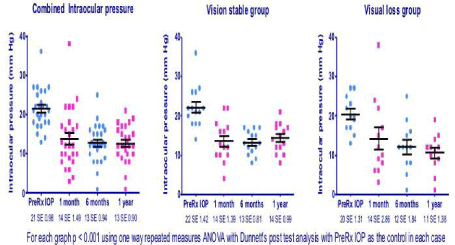
Figure 2:
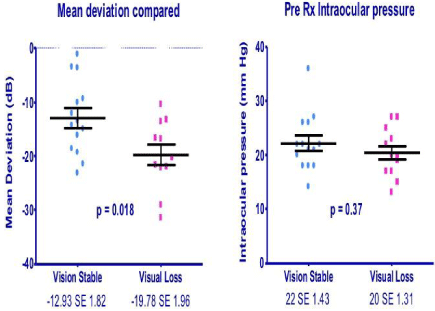
Figure 3:
All the patients stayed on the same pre-treatment glaucoma medications.
2 patients (13%) of the group that maintained visual acuity required 2 sessions of TSCP whereas 4 patients (33%) of the group that lost visual acuity required 2 sessions of TSCP for satisfactory reduction of their IOPs. The pre-treatment visual acuities in these cases that were included in the analysis were their visual acuities prior to their second session of TSCP.
The pre-treatment visual fields showed mean deviation values of -12.93 (SE 1.82) in the group maintaining visual acuity and -19.17 (SE 1.96) in those who lost vision (P=0.018 unpaired t-test), indicating more advanced visual field loss in the latter group (Figure 3).
In terms of complications, 3 patients (11%) of patients developed hypotony, 1 in the group that maintained visual acuity whose IOP eventually recovered to 15mmHg, the other 2 were in the group that lost visual acuity (one from 6/9 to HM with final IOP of 4mmHg, the other from 6/9 to 6/18 with final IOP of 1mmHg.). There were no cases of persistent uveitis or hyphaema or pthisis.
Discussion
The efficacy and safety of the 810nm diode laser in treatment of refractory glaucomas have been well documented in the literature [1-9]. However, the most appropriate laser settings are not known. Egbert et al [13] conducted a prospective study which randomised subjects to two different laser settings found no difference in outcome between the two groups. Earlier, Krott et al [14] attempted to find the correlation between energy applied during TSCP and outcome to no avail.
TSCP appeared to be safer compared to the older neodymium: YAG (Nd: YAG) lasers [20,21]. Loss of vision remains a cause for concern following cyclodestructive procedures. The reason for loss of visual acuity after cycloablation is unclear. Different postulates for the cause of visual deterioration included inflammation, hypotony, macular oedema, or progression of the glaucoma [23-26].
Few studies have looked at changes to visual acuities following TSCP as primary treatment. Ansari et al [10] conducted a retrospective review of 23 eyes with POAG treated with TSCP with mean follow up of 12.5 months and reported only 13% (n=3) of patients with worse final visual acuities which was attributed to cataract (n=2) and progression of GON (n=1). Wilensky et al [15] performed a retrospective case not review of 21 eyes concluded that visual acuity was preserved in most eyes with VA better than 20/80. 3 out of 21 eyes (14%) had deterioration of more than 3 Snellen lines. Egbert et al [13] reported reduction in visual acuity in 23% of patients with a complete follow up period of only 3 months in 78% of patients in Ghana. They found the same percentage reduction in fellow eyes treated on medication alone and also concluded that eyes with good pretreatment VA (20/60 or better) ran less risk of rapid loss of VA compared to eyes with worse VA. These studies advocated that the use of TSCP could be extended to eyes with good visual acuities. In comparison, Pokroy et al [19] conducted a retrospective review of 25 eyes with POAG and found 36% loss of 2 or more logMAR lines despite the relative lack of hypotony and pthisis. Other studies [1-3,8,10,13,16-18,21,27-31] with a mixture of POAG and secondary glaucomas have reported loss of visual acuity ranging from 12% to 53%. All these case series had variable definitions of success of treatment and laser parameters employed and most had patients with advanced glaucoma and poor visual acuities. In our study, 44% of patients with pre-treatment visual acuity of 6/12 had deterioration of vision of more than 1 Snellen line. We found more correlation between pre-treatment visual field loss and subsequent loss of VA suggesting that visual loss was more likely in patients with more advanced visual field loss. It may be that the natural history of these eyes was such that progression of GON would have occurred with or without TSCP. Pokroy et al [19] found that eyes with better visual acuity seemed less susceptible to vision loss, which was not the case in our study. More recently, Zhekov et al [36] reported that 83.6% of their patients retained or even had improvement in their visual acuity following TSCP used as primary surgical intervention for refractory glaucoma. It is noteworthy that their patients had mean deviation pre treatment of -8.74dB, which represented mild visual field loss pretreatment.
It is noteworthy that reduction in visual acuity is not specific to TSCP but also a feature of conventional procedures such as incisional drainage surgery and argon laser trabeculoplasty (ALT) [32]. The rate of visual field progression to blindness varies widely with treatment [33-35]. We do not know of the natural history i.e. the rate of progression without treatment.
This study is limited by its retrospective nature and small size but nevertheless provided some insight into the effect of TSCP on visual acuities, especially in patients with good pre-treatment visual acuities. A more quantitative comparison of visual acuity could be obtained by using LogMAR visual acuities. A randomised controlled trial that compares the TSCP, natural history and the current gold standard of augmented filtration surgery would be needed to make a fair comparison but such a study would be difficult to perform. We recommend that caution should be exercised in using TSCP in patients with good pre-treatment visual acuities; in particular those with advanced visual field loss and this should be included in the pretreatment counselling.
References
- Brancato R, Carassa RG, Bettin P. Contact transscleral cyclophotocoagulation with diode laser in refractory glaucoma. Eur J Ophthalmol. 1995; 5: 32-39.
- Kosoko O, Gaasterland DE, et al. Long term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. Ophthalmology. 1996; 103: 1294-1302.
- Bloom PA, Tsai JC, et al. “Cyclodiode”: trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology. 1997; 104: 1508-1519.
- Gupta N, Weinreb RN. Diode laser transscleral cyclophotocoagulation. J Glaucoma. 1997; 6: 426-429.
- Spencer AF, Vernon SA. “Cyclodiode”: results of a standard protocol. Br J Ophthalmol. 1999; 83: 311-316.
- Walland M J. Diode laser cyclophotocoagulation: longer term follow up of a standardized treatment protocol. Clinical and Experimental Ophthalmology. 2000; 28: 263-267.
- Pucci V, Marchini G, et al. Transscleral diode laser photocoagulaction in refractory glaucoma. Ophthalmologica. 2001; 215: 263-266.
- Murphy CC, Burnett CA, et al. A two centre study of the dose-response relation for transscleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthlamol. 2003; 87: 1252-1257.
- Iliev ME, Gerber S. Long-term outcome of trans-scleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol. 2007; 91: 1631-1635.
- Ansari E, Gandhewar J. Long-term efficacy and visual acuity following transscleral diode laser photocoagulation in cases of refractory and non-refractory glaucoma. Eye. 2007; 21: 936-940.
- Grueb M, Rohrbach JM. Bartz-Scmidt KU, et al. Transscleral diode laser cyclophotocoagulation as primary and secondary surgical treatment in primary open-angle and pseudoexfolicative glaucoma. Long term clinical outcomes. Graefe’s Arch Clin Exp Ophth. 2006; 224: 1293-1299.
- Kramp K. Vick HP, Guthoff R. Transscleral diode laser contact cyclophotocoagulation in the treatment of different glaucomas, also as primary surgery. Graefe’s Arch Clin Exp Ophth. 2003; 240: 689-703.
- Egbert PR, Fiadoyor S, Budenz DL, et al. Diode laser transscleral cyclophotocoagulation as a primary surgical treatment for primary open angle glaucoma. Arch Ophthalmology. 2001; 119: 345-350.
- Krott R, Diestelhost M, Zollweg M, et al. Dose-response relationship of trans-scleral contact cyclophotocoagulation. Ophthalmologe. 1997; 94: 273-276.
- Wilensky J, Kammer J. Long-term visual outcome of transscleral laser cyclotherapy in eyes with ambulatory vision. Ophthalmology. 2004; 111: 1389-1392.
- Yap-Veloso MIR, Simmons RB, Echelman DA et al. Intraocular pressure control after contact transscleral diode cyclophotocoagulation in eyes with Intractable Glaucoma. J Glaucoma. 1998; 7: 319-328.
- Gaasterland DE, Pollack IP. Initial experience with a new method of laser transscleral cyclophotocoagulation for ciliary ablation in severe glaucoma. Trans Am Ophthalmol Soc. 1992; 59: 225-246.
- Threlkeld AB, Johnson MH. Contact transscleral diode cyclophotocolaculation for refractory glaucoma. Journal of Glaucoma. 1999; 8: 3-7.
- Pokroy R, Greenwald Y, Pollack A, et al. Visual loss after transscleral diode laser cyclophotocoagulation for primary open-angle and neovascular glaucoma. Ophthalmic Surgery, Lasers & Imaging. 2008; 39: 22-29.
- Schuman JS, Bellows AR, Chingleton BJ, et al. Contact transscleral Nd: YAG laser cyclophotocoagulation: mid-term results. Ophthalmology. 1992; 99: 1089-1094.
- Goldenberg-Cohen N, Bahar I, Ostashinski M, et al. Cyclocryotherapy versus transscleral diode laser cyclophotocoagulation for uncontrolled intraocular pressure. Ophthalmic Surg Lasers Imaging. 2005; 36: 272-279.
- Bellows AR, Grant WM. Cyclocryotherapy of chronic open angle glaucoma in aphakic eyes. Am J Ophthalmol. 1978; 85: 615-621.
- Dickens CJ, Nguyen N, Mora JS, et al. Long-term results of noncontact transscleral Neodymium:YAG cyclophotocoagulation. Ophthalmology. 1995; 102: 1777-1781.
- Trope GE, Ma S. Midterm effects of neodymium: YAG transscleral cyclocoagulation in glaucoma. Ophthalmology. 1990; 97: 73-75.
- Hampton C, Shields MB, Miller KN, et al. Evaluation of protocol for transscleral neodymium: YAG cyclophotocoagulation in one hundred patients. Ophthalmology. 1990; 97: 910-917.
- Benson MT, Nelson ME. Cyclocryotherapy: a review of cases over a ten year period. Br J Ophthalmol. 1990; 74: 103-105.
- Schlote T, Derse M, Zierhut M. Transscleral diode laser cyclophotocoagulation for the treatment of refractory glaucoma secondary to inflammatory eye diseases. Br J Ophthalmol. 2000; 84: 999-1003.
- Lai JS, Tham CC, Chan JC, Lam DS. Diode laser transscleral cyclophotocoagulation as primary surgical treatment for medically uncontrolled chronic angle closure glaucoma: longterm clinical outcomes. J Glaucoma. 2005; 14: 114-119.
- Noureddin BN, Zein W, Haddad C, et al. Diode laser transscleral cyclophotocoagulation for refractory glaucoma: 1 year follow-up of patients treated using an aggressive protocol. Eye. 2006; 20: 329-335.
- Rebolleda G, Munoz FJ, Murube J. Audible pops during cyclodiode procedures. J Glaucoma. 1999; 8: 177-183.
- Hauber F, Scherer W. Influence of total energy delivery on success rate after contact diode laser transscleral cyclphotocoagulation: a retrospective case review and meta-analysis. J Glaucoma. 2002; 11: 329-333.
- The AGIS Investigators. The advanced glaucoma intervention study (AGIS): 4. Comparison of treatment outcomes with race. Ophthalmology. 1998; 105: 1146-1164.
- Quigley HA, Tielsch JM, Katz J, et al. Rate of progression in open-angle glaucoma estimated from cross-sectional prevalence of visual field damage. Am J Ophthalmol. 1996; 122: 355-363.
- Hattenhauer MG, Douglas DH, Ing HH, et al. The probability of blindness from open-angle glaucoma. Ophthalmology. 1998; 105: 2099-2104.
- Rasker MTE, Enden A, Bakker D, Hoyng P. Rate of visual field loss in progressive glaucoma. Arch Ophthalmol. 2000; 118: 481-488.
- Zhekov I, Janjua R, Shahid H, et al. A retrospective analysis of long term outcomes following a single episode of transscleral cyclodiode laser treatment in patients with glaucoma. BMJ Open. 2013; 3: e002793.