
Research Article
Austin J Clin Ophthalmol. 2018; 5(1): 1089.
Is Amblyopia Really Rare in Brown Syndrome?
Yaman D¹, Yuzbasioglu S¹, Onursever N¹ and Yulek F²*
¹MD, Ophthalmology Department, Yildirim Beyazit University Medical Faculty, Ankara Atatürk Education and Research Hospital, Turkey
²Prof Dr, Ophthalmology Department, Yildirim Beyazit University Medical Faculty, Ankara Atatürk Education and Research Hospital, Turkey
*Corresponding author: Fatma Yulek, Gülden Sokak No: 13/1 Kavaklidere, Ankara, Turkey
Received: February 19, 2018; Accepted: March 14, 2018; Published: March 22, 2018
Abstract
Purpose: Evaluating the sensory status and accompanying horizontal and vertical deviations in Brown syndrome cases.
Method: Visual acuity, affected eye, refractive error, presence of amblyopia, horizontal and vertical deviations were evaluated retrospectively in twenty one Brown syndrome cases observed in Ankara Ataturk Training and Research Hospital Strabismus Unit between 2006 and 2017.
Result: The mean follow-up period was 4.31±3.88 years for twenty-one patients in this study. The left eye was affected in 47.6% (n=10) of the cases and 9.5% (n=2) were bilateral. Amblyopia was observed in 28.6% (n=6) of the patients. In four patients, amblyopia was on the same side as Brown syndrome. Thirteen patients (61.9) were orthophoric. Anisometropia was detected in five (23.8%) patients Anisometropia and strabismus were observed together in four of these five patients.
Conclusion: Brown syndrome patients are mostly orthophoric while accompanying horizontal and vertical deviations were present in 38.1% of the cases and amblyopia in six patients (28.6%). Detection of deviations and possible amblyopia secondary to deviation is critical in Brown syndrome because of their impact on binocular sensory development.
Keywords: Amblyopia; Anisometropia; Brown syndrome
Introduction
Brown syndrome is a rare ocular motility disorder, characterized by restriction of elevation in adduction due to abnormalities in the trochlea or superior oblique tendon sheath [1]. This syndrome may be congenital, acquired, intermittent or constant. Although its etiology remains uncertain, various causes have been associated with Brown syndrome [2].
The reason of congenital Brown syndrome is an abnormality of the superior oblique tendon or trochlea, while acquired Brown sydrome is caused by various reasons including infection, scleritis, systemic inflammatory disease such as rheumatoid arthritis and systemic lupus erythematosus, trauma, orbital metastatic deposits in the extraocular muscles, mucopolysaccharidosis, glaucoma drainage device implantation, scleral buckling, frontal sinusitis, orbital wall fracture or sinus surgery [2-10].
Variable clinical features can be seen in Brown Syndrome such as a positive forced duction test, down-shoot in adduction, V pattern exotropia in the up gaze, abnormal head position including chin up and contralateral face turn, and hypotropia in primary position [3].
Amblyopia, a neurological developmental disorder that affects 3-5% of the population [13,14], is characterized by monocular visual acuity loss in an anatomic healthy eye, despite optimal refractive correction. There are several causes for amblyopia including uncorrected refractive error, anisometropia, strabismus and visual deprivation (such as cataract, opaque cornea, complete ptosis, prolonged uncontrolled patching) [15-19].
Several studies have reported that amblyopia is not common in Brown syndrome because of rare incidence of suppression [11,12]. Although some case reports are available, there is limited literature on clinical finding with accompanying anisometropia and deviations in Brown syndrome cases. We aimed to report evaluation of sensory status and accompanying horizontal-vertical deviations in Brown syndrome cases.
Materials and Methods
The medical records of twenty-one patients that visited Ataturk Training and Research Hospital Strabismus Unit from 2006 to 2017 with the diagnosis of Brown syndrome were retrospectively reviewed. Congenital and trauma induced acquired Brown syndrome patients having no ocular and sinus surgery, with a minimum of six-month follow-up was included in this study. Patients with amblyopia due to ocular media opacity, retinal disorders and systemic and autoimmune diseases, tumors, orbital and sinus surgery history were excluded from the study. The medical records of the patients including demographic features, affected eye, best-corrected visual acuity, presence of amblyopia, the amount of horizontal-vertical deviations and refractive errors were reviewed. Visual acuity was evaluated by Snellen chart. For statistical analysis the visual acuity was converted to LogMAR. Amblyopia was defined as a difference of two or more lines between the best-corrected visual acuity of two eyes. Anisometropia was accepted as the difference of 1.50D or more (sphere or cylinder) between the two eyes. The amount of manifest deviation was assessed by using prism cover test. Statistical analysis of the data obtained in this study was performed using the Statistical package for Social Sciences (SPSS) 20 program. Categorical data were analyzed using frequency and percentages. The comparisons between eyes with and without Brown syndrome were made by Mann Whitney U test. The p values less than 0.05 were accepted as statistically significant.
Results
The mean follow-up period was 4.31 ± 3.88 years for twenty-one patients. Seventeen cases (81%) were female and the mean age was 10.62 ± 5.07 years. The left eye was affected in 47.6% (n=10) of the cases and 9.5% (n=2) of the cases were bilateral.
The mean best corrected visual acuity was 0.91 ± 0.16 and 0.91 ± 0.19 for the eye with Brown syndrome and the other eye respectively (p=0.23).
The mean corrected spherical refraction error was +0,80 ± 2,02 in the eye with Brown syndrome; +0.06 ± 1.41 for the other eye without Brown syndrome (p=0.14) while corrected astigmatic refractive error was -0.08 ± 1.28 for the eye with Brown syndrome and-0.36 ± 1.24 for the other eye (p=0.35). Ten patients (47.6%) had hypermetropic astigmatism, five patients (23.8%) had myopic astigmatism 2 patients (9.5%) had hyperopia and 1 patient (4.8%) had myopia. Three of all patients (14.3%) had no refractive error (Figure 1).
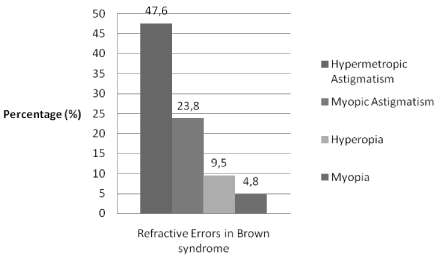
Figure 1: Distribution of refractive errors in Brown syndrome.
Amblyopia was observed in 28.6% (n=6) of the patients. The mean best corrected visual acuity of amblyopic eyes was 0.56 ± 0.26. In four patients, amblyopia was on the same side as Brown syndrome (Figure 2). Amblyopia and deviations (two of the patients have esotropia) were seen in three patients on the same side as Brown syndrome.
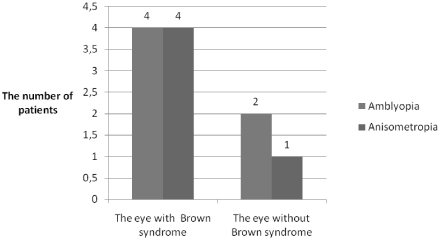
Figure 2: Distribution of amblyopia and anisometropia in eyes with and
without Brown syndrome.
Anisometropia was detected in five (23.8%) patients and four patients had high refractive error in the eye with Brown syndrome. Amblyopia due to anisometropia and deviations was observed in four of these five patients (Figure 2).
61.9% (n=13) of the patients were orthophoric while deviations were seen in 38.1% (n=8) of them. Isolated hypotropia, hypotropia with exotropia, esotropia and exotropia was observed in 14.3% (n=3), 4.8% (n=1), 9.5% (n=2), 9.5% (n=2) 0f the cases respectively (Figure 3). In addition, V pattern deviation in upward gaze was observed in 23.8% (n=5) of the patients.
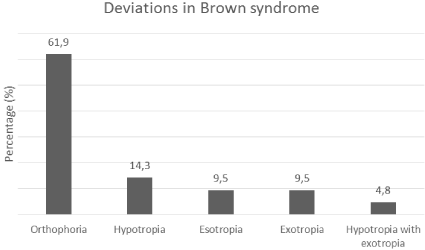
Figure 3: General distribution of deviations in Brown syndrome.
The mean horizontal near and distance deviations were 15Δ (12- 18 Δ) and 11Δ (4-20 Δ) respectively. The mean vertical deviation was 15Δ (12-20 Δ) at near and 13Δ (10-16 Δ) at distance.
Discussion
We investigated clinical findings 21 cases with Brown syndrome. We observed that amblyopia was present in 28.6% (n=6) of patients and horizontal or vertical deviations in 38.1% of the patients with Brown syndrome.
The most common refractive error that can induce amblyopia is uncorrected hyperopia which is more common due to the natural distribution of refractive error at the young age group. Similarly, hyperopic astigmatism (47.6) was most common refractive error in this study.
Nonetheless there are studies that offer different views on the effect of anisometropia on amblyopia. According to several studies, the depth of amblyopia increases as the amount of anisometropia increases [20,21]. But there are also studies advocating that there is no association between anisometropia and amblyopia in Brown syndrome because of rare incidence of suppression [11,12] whereas severe amblyopia was presented in one case report [25]. Similarly, Brown et al. have reported that amblyopia and refractive errors have no significant importance in Brown syndrome. Although in the study of Sekeroglu et al 7 patients (15.9%) had amblyopia and 14 patients (31.8%) had anisometropia [26], anisometropia and strabismus were not reported as significant amblyogenic factors in Brown syndrome.
Strabismus causes inhibition in the retinocortical pathway and in vivo studies have shown decreased metabolic activation of the visual cortex [27]. Previous studies have reported that strabismic amblyopes have denser suppression than anisometropic amblyopes [14-28].
According to the study of Sekeroglu et al exotropia was found to be the most common strabismus in cases with Brown syndrome. However, in our study we found that anisometropia and strabismus were seen in Brown syndrome in association with amblyopia.
Similar to previous studies there were more female patients (81%) in this study [12]. We observed that the left eye was involved in 47.6% (10/21 patients) of our cases and 9.5% (n=2) of the patients were bilateral while the right eye was involved (70/126 patients) in the study of Wright et al [12]. This difference may depend on the number of patients as well as the possible differences in the populations studied.
We have some limitations in this study. Since it is a retrospective study some information related to sensorial status is missing. Patients without symptoms are usually lost to follow up resulting in variable follow up information. Limited size of the study group should also be considered while interpreting the results. The number of patients should be increased to confirm the high prevalence of amblyopia and anisometropia in Brown syndrome.
Conclusion
In conclusion we have observed that Brown syndrome patients are mostly orthophoric, although horizontal and vertical deviations and amblyopia can be seen in contrast to previous reports presenting lower incidence [11,12]. We think that the detection of symptoms such as deviations, anisometropia and amblyopia is important for early treatment and normal binocular sensory development in Brown syndrome patients.
References
- Wilson ME, Eutis HS, Parks MM. Brown’s syndrome. Surv Ophthalmol. 1989; 34: 153–172.
- Shin KH, Paik HJ, Chi M. Acquired Brown syndrome treated with traction of superior oblique tendon. J Craniofac Surg. 2016; 27: 176–178.
- Jae HY, Won H, Ji WL, et al. Three Cases of Acquired Simulated Brown Syndrome after Blowout Fracture Operations. Arch Plast Surg. 2015; 42: 346-350.
- Coats DK, Paysse EA, Orenga-Nania S. Acquired Pseudo-Brown’s syndrome immediately following Ahmed valve glaucoma implant. Ophthalmic Surg Lasers. 1999; 30: 396–397.
- Park BC, Kim YH, Kim TG, et al. Treatment of posttraumatic facial deformity patient with Brown’s syndrome: case report. J Korean Cleft Palate-Craniofac Assoc. 2010; 11: 33-36.
- Booth-Mason S, Kyle GM, Rossor M, et al. Acquired Browns Syndrome: an unusual cause. Br J Ophthalmol. 1985; 69: 791–794.
- Killian PJ, McClain B, Lawless OJ. Brown’s syndrome: An unusual manifestation of rheumatoid arthritis. Arthritis Rheum. 1977; 20: 1080-1084.
- Pandey PK, Chaudhuri Z, Bhatia A. Extraocular muscle cysticercosis presenting as Brown’s syndrome. Am J Ophthalmol. 2001; 131: 526–527.
- Bradbury JA, Martin L, Strachan IM. Acquired Brown’s Syndrome associated with Hurler-Scheie’s Syndrome. Br J Ophthalmol. 1989; 73: 305–308.
- Lauer SA, Sauer H, Pak SM. Brown’s syndrome diagnosed following repair of an orbital roof fracture: a case report. J Craniomaxillofac Surg. 1998; 4: 20-22.
- Von Noorden GK, Campos EC. Binocular Vision and Ocular Motility- Theory and Management of Strabismus. 6th St. Louis: Mosby. 2002; 246-297.
- Brown HW. True and simulated superior oblique tendon sheath syndrome. Doc Ophthalmol. 1973; 34: 123–136.
- Attebo K, Mitchell P, Cumming R, et al. Prevalence and causes of amblyopia in an adult population. Ophthalmology. 1998; 105: 154–159.
- Webber AL, Wood J. Amblyopia: Prevalence, natural history, functional effects and treatment. Clinical & Experimental Optometry. 2005; 88: 365–375.
- Flom MC, Neumaier RW. Prevalence of amblyopia. Public Health Rep. 1966; 81: 329–341.
- Brendan TB, Arthur B, T RC. The Relationship between Anisometropia and Amblyopia. Prog Retin Eye Res. 2013; 36: 120–158.
- Eileen EB. Amblyopia and Binocular Vision. Prog Retin Eye Res. 2012; 33: 67–84.
- Wang B, Naidu RK, Qu X. The use of rigid gas permeable contact lenses in children with myopic amblyopia: A case series. Cont Lens Anterior Eye. 2017.
- Leon A, Donahue SP, Morrison DG, et al. The age-dependent effect of anisometropia magnitude on anisometropic ambliyopia severity. JAAPOS. 2008; 12: 150–156.
- Copps LA. Vision in anisometropia. Am J Ophthalmol. 1944; 27: 641–644.
- Barrett BT, Bradley A, Candy TR. The relationship between anisometropia and amblyopia. Prog Retin Eye Res. 2013; 36: 120–158.
- Horwich H. Anisometropia Amblyopia. American Orthoptic Journal. 1964; 14: 99–104.
- Helveston EM. Relationship between degree of anisometropia and depth of amblyopia. Am J Ophthalmol. 1966; 62: 757–759.
- Donahue SP. Relationship between anisometropia, patient age, and the development of amblyopia. Am J Ophthalmol. 2006; 142: 132–140.
- Bolutife AO. Brown syndrome with severe amblyopia: a case report from Africa. Pan African Medical Journal. 2015; 20: 56.
- Sekeroglu HT, Muz E, Sanac AS, et al. Amblyopia and sensory features at initial presentation of Brown syndrome: an issue to recognize. Eye. 2013; 27: 515-518.
- Von Noorden GK, Crawford ML. The lateral geniculate nucleus in human strabismic amblyopia. Invest Ophthalmol Vis Sci. 1992; 33: 2729-2732.
- Narasimham S, Harrison ER, Giaschi DE. Quantitative measurement of interocular suppression in children with amblyopia. Vision Res. 2012; 66: 1–10.