
Research Article
Austin J Clin Ophthalmol. 2018; 5(2): 1091.
Retinal Vein Occlusion in Cotonou
Odoulami L*, Savage Y, Alamou S, Sounouvou I, Tchabi S and Doutetien C
Department of Ophtalmology, University of Abomey- Calavi, Benin
*Corresponding author: Odoulami L, Department of Ophthalmology, University of Abomey-Calavi, 03 BP 2915 Benin
Received: March 15, 2018; Accepted: April 17, 2018; Published: April 24, 2018
Abstract
Objectives: To study the epidemiological, clinical, therapeutic and evolutionary features of RVO.
Materials and Methods: This was a descriptive and analytical crosssectional study with retrospective collection. All patient records with RVO that visited 4 ophthalmic clinics in Cotonou over a 32 months period were reviewed. Data obtained were analyzed with the Epi-Data Analysis software version 2.2.2.182 and a p<0.05 considered significant.
Results: Of the 96047 patients, 30 (0.03%) had RVO. The most represented patients were over 55 years old; the mean age was 58 ± 12 years. Sex ratio was 1.5. All patients presented on account of visual loss. Bilateral involvement occurred in 4 (13.3%) patients. Hypertension, glaucoma and diabetes were the most common disorders in our patients. There were 15 eyes (44.1%) with CRVO, 14 eyes (41.2%) with BRVO and 5 eyes (14.7%) with HRVO.67.6% of eyes had visual acuity less than 3/60 at presentation. Macular edema was the most common complication. Almost half (47.1%) of the affected eyes have not been treated. Intravitreal anti-VEGF, laser photocoagulation and intravitreal corticosteroids were the most used therapeutic means. The improvement in visual acuity after treatment was barely noticeable.
Conclusion: RVO are an important cause of visual acuity drop with a predominance of CRVO and BRVO. Early and appropriate treatment and treatment of risk factors could improve their functional prognosis.
Keywords: Retinal vein occlusion; Cotonou; Decrease in visual acuity; Risk factors
Abbreviations
BRVO: Branch Retinal Vein Occlusion; CRVO: Central Retinal Vein Occlusion; HRVO: Hemiretinal Vein Occlusion; INV: Iris Neovascularization; MO: Macular Edema; NVG: Neovascular Glaucoma; POAG: Primitive Open Angle Glaucoma; RD: Retinal Detachment; RNV: Retinal Neovascularization; RVO: Retinal Vein Occlusion; SRD: Serous Retinal Detachment; VA: Visual Acuity; VEGF: Vascular Endothelial Growth Factor; VH: Vitreous Hemorraghe
Introduction
Retinal vein occlusions (RVO) are one of the most common causes of retinal vascular disease in adults (behind diabetic retinopathy), and a common cause of decreased visual acuity [1] making the severity of the disease; their prevalence increases considerably from the age of 60 [2-4]. The related risk factors are found in the Virchow triad, which combines the abnormalities of the container, the anomalies of the contents and the haemodynamic component [5].
Described since 1855 and topic of more than 3,000 publications mainly derived from studies in Caucasians and more recently in other ethnic groups [1], retinal vein occlusions have been little researched in melanoderms.
In Benin, no study has focused on retinal vein occlusions. It is therefore appropriate to take stock of the epidemiological profile, the clinical, therapeutic and evolutionary aspects in order to better know this pathology on the one hand and to take care of it on the other hand.
Materials and Methods
This was a descriptive and analytical cross-sectional study with retrospective collection. All patient records with RVO and fluorescein angiography, in 4 ophthalmic clinics in Cotonou from January 1st, 2015 to August 31, 2017 were reviewed. Data obtained were analyzed with the Epi-Data Analysis software version 2.2.2.182 and a p<0.05 considered significant.
Collected data included demographic characteristics, risk factors, presenting visual acuity, IOP, clinical diagnosis, ocular complications, treatments offered and outcomes.
The diagnosis of RVO was funduscopic and was guided by venous dilatation and/or tortuosity, superficial or deep haemorrhages, cotton wool spots, retinal edema associated or not with macular involvement. According to their location, we distinguished CRVO with these signs widespread scattered to the retinal field, BRVO with these signs occuring within one retinal sector and HRVO with signs were present in the upper or lower retinal half. Confirmatory investigations as fundus fluorescein angiography and/or macular optical coherence tomography were helpful for the diagnosis of complications which are macula edema, sub-retinal detachment, vitreous haemorrhages, retinal neovascularization.
Results
Out of 96047 patients seen in these 4 clinics, there were 30 (0.03%) cases of documented RVO during the study period. The mean age was 58 ± 12 years (range 35-77 years); the most represented patients were over 55 years old. There were 18 males (60%) and 12 females (40%) giving a sex-ratio of 1.5. more than half (56.7%) of the patients consisting of traders (30%), civil servants (16.7%) and artisan (10%) (Figure 1).
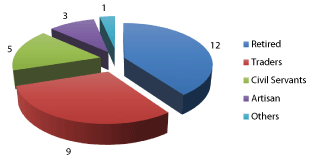
Figure 1: Occupational Distribution of Patients.
All patients presented on account of visual loss. Hypertension, glaucoma and diabetes were the most common antecedents in our patients. Bilateral involvement occurred in 4 (13.3%) patients giving a total of 34 eyes. No matter the RVO’s type, 67.6% of eyes had visual acuity less than 3/60 at presentation (Figure 2). There were 15 eyes (44.1%) with CRVO, 14 eyes (41.2%) with BRVO and 5 eyes (14.7%) with HRVO. There were 13 (38.2%) eyes with both form of RVO, 8 (23.5%) eyes with ischemic RVO while 6 (17.6%) eyes have non ischemic RVO. 71.4% of BRVO was found in the superotemporal branch while lower retinal half was the most common (60%) location in the eyes with HRVO. Macular edema was the most common complication (Figure 3).
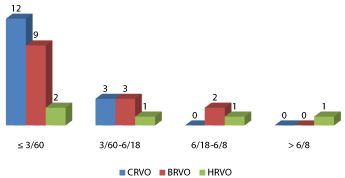
Figure 2: Visual Acuity and Type of Retinal Vein Occlusion.
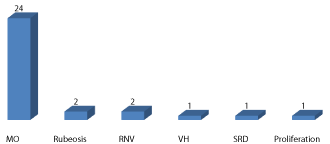
Figure 3: Complications of RVO.
Table 1 shows the relationship between RVO and hypertension. 7 (23.3%) of patients with CRVO were hypertensive, where as 9 of patients with BRVO were hypertensive. The difference was not statistically significant (p = 0.109). Table 2 shows the association between RVO and glaucoma. 6(20%) patients with CRVO had glaucoma while 4 (13.3%) with BRVO had glaucoma. This was however not statistically significant (p = 0.807). The relationship between RVO and diabetes is illustrated in Table 3. Diabetes was present in 2 (6.7%) patients who presented with CRVO while 6 (20%) patients with BRVO presented with diabetes. The difference was not statistically significant (p = 0.146).
Hypertension (n (%))
p-value
Absent
Present
Total
CRVO
6(20)
7(23.3)
13(43.3)
0.109
BRVO
3(10)
9(30)
12(40)
HRVO
4(13,3)
1(3.3)
5(16.7)
Table 1: Association between RVO and Hypertension.
Glaucoma (n (%))
p-value
Absent
Present
Total
CRVO
7(23.3)
6(20)
13(43.3)
0.807
BRVO
8(26.7)
4(13.3)
12(40)
HRVO
3(10)
2(6.7)
5(16.7)
Table 2: Association between RVO and Glaucoma.
Diabetes (n (%))
p-value
Absent
Present
Total
CRVO
11(36.7)
2(6.7)
13(43.3)
0.146
BRVO
6(20)
6(20)
12(40)
HRVO
4(13.3)
1(3.3)
5(16.7)
Table 3: Association between RVO and Diabetes.
Almost half (47.1%) of the affected eyes have not been treated. Intravitreal anti-VEGF, laser photocoagulation and intravitreal corticosteroids were the most used therapeutic means (Table 4).
Treatments
n
%
Anti-VEGF
9
50.0
Laser Photocoagulation
7
38.9
Corticosteroids
6
33.4
Rheological treatment (Vastarel*)
5
27.8
Antiglaucoma medications
4
22.2
Retrobulbar alcohol
2
11.1
Platelet antiaggregants
1
5.6
Evisceration
1
5.6
Table 4: Received Treatments.
The mean duration of post-treatment follow-up was 18 ± 17 months (range 2-102 months). The improvement in visual acuity after treatment was barely noticeable (Table 5) while macular edema, which was the most common complication, almost receded after treatment (Table 6).
VA before treatment
VA after treatment
= 3/60
3/60-6/18
6/18-6/8
= 3/60
3/60-6/18
6/18-6/8
CRVO
7
1
0
6
1
1
BRVO
4
2
2
5
1
2
HRVO
2
0
0
0
0
2
Total
13
3
2
11
2
5
Table 5: RVO and visual acuity before and after treatment.
Before treatment
After treatment
MO
VH
INV
RNV
SRD
MO
VH
INV
NVG
RNV
RD
CRVO
5
1
1
2
1
0
0
0
1
0
0
BRVO
6
0
1
0
0
1
1
1
0
0
2
HRVO
2
0
0
0
0
0
0
0
0
0
0
Total
13
1
2
2
1
1
1
1
1
0
2
Table 6: RVO and complications before and after treatment.
Discussion
RVO accounted for 0.03% of the pathologies in our study. In a meta-analysis of pooled daata from the United States, Europe, Asia and Australia [1], this prevalence varies between 0.3 and 1.6%. Western and Asian studies [6,7,10] have found very high prevalence compared to our serie. Studies carried out in Nigeria [8,9] are mainly incidence studies. RVO would be relatively rare in our population. Nevertheless, these results can not be representative of the population because the study was a hospital based one and the fact that in our context, not all patients with RVO got fluorescein angiography. A prospective population based study over several years would allow us to have more reliable results.
The mean age of our patients was 58 ± 12 years (range 35-77 years). It is the same at the university of Port Harcourt in Nigeria [8] who reported a mean age of 54.8 ± 10.1 years range from 38 to 73 years. Other authors have reported a higher mean age than ours: Uhumwangho et al. [9] in Nigeria in 2016: 62.7 ± 10.4 years (43-87 years) ; Thapa et al. [12] in Nepal in 2010: 61.1 ± 12.3 years. 63.3% of our patients were over 55 years old. This agrees with the literature that describes a high frequency of RVO in the older population [1] with more than 90% of cases of RVO occuring in the age group over 50 years [13].
There was a male preponderance in this study. Ponto et al. [6] in Germany in 2015 also report a male preponderance as well as Uhumwangho et al. [9]. On the other hand, other nigerian studies [8, 14] report a female preponderance related to the use of oral contraceptives and hormone replacement therapy. Male preponderance is effective as described in the literature [12,15,16]. However, a meta-analysis [1] find no difference between both sexes.
All patients presented on account of decrease in visual acuity. In the literature [5,17,18], the decrease in visual acuity is the most common functional sign in retinal venous occlusions and the main circumstance of discovery.
The most common antecedents in our series were hypertension, POAG and diabetes with respectively 56.7%, 40% and 30% of cases. Adenuga et al. [19] in Nigeria in 2015, along with Uhumwangho et al. [9] found in their series hypertension (70%), diabetes (45%) and glaucoma (22.7%).Two cases of hyperlipidemia were found in the Uhumwangho et al. [9] against one in ours.This testifies to the effectiveness of the factors that act according to Virchow in the pathogenesis of RVO.
Unilateral involvement occurs in 26 cases (86.7%) and bilateral in 4 cases (13.3%). Fiebai et al. [8] as well as Uhumwangho et al. [9] reported 26% and 10%, respectively, of bilateral involvement. Unilateral involvement would be the rule in RVO, but bilateralization is possible in 7 to 15% of cases, with an average time to reach the eye of 19 months. It is conventional to observe that the involvement of the second eye is more severe than the first [5].
No matter the type of RVO, more than half of the affected eyes (67.6%) had a visual acuity less than 3/60 at presentation, of which half had CRVO. This same observation was made by Fiebai et al. [8] and Vonor et al. [20]. These figures reflect the drop of visual acuity in RVO. This drop is even more important when it occurs in CRVO.
We found 15 eyes (44.1%) with CRVO, 14 eyes (41.2%) with BRVO and 5 eyes (14.7%) with HRVO.A greater proportion of CRVO was reported by Uhumwangho et al. [9] with 68.2% CRVO, 18.2% HRVO and 13.6% BRVO and by Fiebai et al. [8] with 74% CRVO and 26% BRVO. This preponderance was also found in Lomé in 1999 by Ayena et al. [15] with 67.64% of cases. On the other hand, Vonor et al. [21], Klein et al. [3], Glacet-Bernard et al. [5], Thapa et al. [12] found that BRVO was more prevalent in their respective series. Our results would be closer to the predominance of CRVO or BRVO, considering, as some authors [22] do, that HRVO can be pooled with CRVO or BRVO.
A greater proportion of eyes with RVO had mixed form (38.2%) in comparison with ischemic or nonischemic form. The most common form in Fiebai et al. [8] was nonischemic (85.2%) followed by ischemic (14.8%). The same observation was made by Vonor et al. [20] with 78.6% nonischemic form and 21.6% ischemic form and by Glacet-Bernard et al. [5] with 77% nonischemic form and 16% ischemic form.This low frequency of nonischemic forms in our study could be explained by a conversion to ischemic form whose rate varies from 10 to 54% according to the authors [5] and the fact that some angiography results did not specify the form of RVO due to the retrospective collection.
In our series, the superior temporal location (71.4%) was the most common in BRVO and the inferior one (60%) was the most common in HRVO. For BRVO, Glacet-Bernard et al. [5], Thapa et al. [12] agree with respectively 62% and 63.9% of superior temporal location. For HRVO, there does not seem to be a predominance of superior or inferior hemiretin damage in the literature [5].
Macular edema was the most common complication (70.6%) followed by rubeosis and retinal neovascularization. Most studies have echoed the same. Thus, the most common complications in the Uhumwangho et al. [9] were macular edema (68.2%), retinal neovascularization (22.7%), and rubeosis (13.6%). In that of Ajayi et al. [14] were macular edema (56.4%), retinal neovascularization (23.1%) and NVG (10.3%).Macular edema is therefore the most common complication of OVR.
In our study, none of the factors (age, sex, hypertension, POAG, diabetes) had a significant association with RVO. Nevertheless, they have been statistically associated with RVO in the literature [1,16,21,23-26]. These non-significant associations in our study may be due to the small size of our sample, which was also the case in nigerian studies [8,9].
Intravitreal anti-VEGF, corticosteroids and laser photocoagulation were the most used therapeutic means with respectively 50%, 33.4% and 38.9% of the treated eyes. These means are more used for the prevention and / or treatment of complications related to RVO. Ajayi et al. [14] reported that 61.53% of the eyes received anti-VEGF, 23.1% antiglaucoma, 11.5% had photocoagulation and 3.8% vitrectomy.
No matter the type of RVO, before and after treatment a greater proportion of eyes had a VA less than or equal to 3/60. Improvement of VA after treatment was barely noticeable. This report could be explained by the funduscopic pattern of RVO in our study with the predominance of mixed and ischemic forms and macular edema. The natural course of the disease has shown that the vision of patients with CRVO will probably worsen or remain unchanged and that patients with poor vision initially have little hope of significant recovery [27]. Macular edema has been reported as the leading cause of vision loss in patients with RVO [28]. The delay for presentation was not given in our study. There is good evidence that early treatment may be beneficial and that the risk of permanent structural damage to the fovea, poor visual prognosis and neovascularization increases with the duration of RVO [1,27,29].
In our study, there were fewer complications after treatment. We could conclude that the treatment was more effective in treating RVO-related complications than RVO themselves.
The treatments available to treat OVR have not really proved effective, leaving just the prevention and treatment of complications as the only strategy to hope for a favorable prognosis in RVO [5].
The retrospective nature of the study was also a limitation as we were compelled to use what information was available in the records.
Conclusion
In our environment, RVO is a rare condition that affects in average a 58-year-old male presenting with risk factors such as hypertension, POAG, and diabetes. These conditions are also serious by their type and form and by macular edema - their most common complication - which is responsible of the drop of visual acuity. They were treated by anti-VEGF for the majority on average 18 ± 17 months without much improvement in visual acuity. A prospective national RVO study over several years would allow us to obtain more reliable results.
References
- Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, et al. The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia and Australia. Ophthalmology. 2010 ; 117: 313–319.e1
- Shahid H, Hossain P, Amoaku WM. The management of retinal vein occlusion: is interventional ophthalmology the way forward? Br J Ophthalmol. 2006; 90: 627-639.
- Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000; 98: 133-141; discussion 141-143.
- Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996; 114: 1243-1247.
- Glacet-Bernard A, Coscas G, Pournaras CJ, et al. Occlusions veineuses rétiniennes. Bulletin des sociétés d’ophtalmologie de France. 2011: 324p
- Ponto KA, Elbaz H, Peto T, Laubert-Reh D, Binder H, Wild PS, et al. Prevalence and risk factors of retinal vein occlusion: the Gutenberg Health Study. J Thromb Haemost. 2015; 13: 1254-1263.
- Yasuda M, Kiyohara Y, Arakawa S, Hata Y, Yonemoto K, Doi Y, et al. Prevalence and systemic risk factors for retinal vein occlusion in a general Japanese population: the Hisayama study. Invest Ophthalmol Vis Sci. 2010; 51: 3205-3209.
- Fiebai B, Ejimadu CS, Komolafe RD. Incidence and risk factors for retinal vein occlusion at the University of Port Harcourt Teaching Hospital, Port Harcourt, Nigeria. Niger J Clin Pract. 2014; 17: 462-466.
- Uhumwangho OM, Oronsaye D. Retinal vein occlusion in Benin City, Nigeria. Niger J Surg. 2016; 22: 17-20.
- Shin YU, Cho H, Kim JM, Bae K, Kang MH, Shin JP, et al. Prevalence and associated factors of retinal vein occlusion in the Korean National Health and Nutritional Examination Survey, 2008-2012: A cross-sectional observational study. Medicine (Baltimore). 2016; 95: e5185.
- Martínez F, Furió E, Fabiá MJ, Pérez AV, González-Albert V, Rojo-Martínez G, et al. Risk factors associated with retinal vein occlusion. Int J Clin Pract. 2014; 68: 871-881.
- Thapa R, Paudyal G, Bernstein PS. Demographic characteristics, patterns and risk factors for retinal vein occlusion in Nepal: a hospital-based case-control study. Clin Exp Ophthalmol. 2010; 38: 583-590.
- JW Yau, P Lee, TY Wong, J Best, A Jenkins. Retinal vein occlusion: An approach to diagnosis, systemic risk factors and management. Intern Med J. 2008; 38: 904–910.
- Ajayi IA, Omotoye OJ, Olumide AK, Alegbeleye TT, Kumolalo F. Demographic Characteristics and Management Challenges of Retinal Vein Occlusion in Ekiti State, Nigeria. Journal of Health Science. 2017; 7: 33-37.
- Ayena DK, Akossou SY, Belo M, Pio M, Agbo RDA, Moumouni I, et al. Résultats thérapeutiques des occlusions veineuses rétiniennes a Lomé après un suivi de 5 ans. Médecine d’Afrique Noire. 2009; 56: pp. 351-355.
- Stem MS, Talwar N, Comer GM, Stein JD. A longitudinal analysis of risk factors associated with central retinal vein occlusion. Ophthalmology. 2013; 120: 362–370.
- Glacet-Bernard A, Soubrane G, Coscas G. Bilan et traitement de l’occlusion de la veine centrale de la rétine. La Lettre du Cardiologue. 2001; 349: 50-54.
- Hatz K, Prünte C. Occlusions veineuses rétiniennes «Nouveaux» et «anciens» traitements d’une affection du ressort de l’ophtalmologie et de la médecine interne. Forum Med Suisse. 2012; 12: 170–174.
- Adenuga OO, Ramyil AV, Odugbo OP, Oyediji FJ. Prevalence, pattern and risk factors for retinal vascular occlusions in a tertiary hospital in Jos, Nigeria. Niger J Med. 2015; 24: 331-336.
- K Vonor, M Santos, KM Amédomé, K Dzidzinyo, N Maneh, KD Ayéna, et al. Les occlusions vasculaires rétiniennes à Lomé. Journal Français d’Ophtalmologie. 2015; 38: e163-e168.
- Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophtalmol. 1994; 1170: 429-441.
- Browning D.J. Classification of Retinal Vein Occlusion. In: Retinal Vein Occlusions. Springer, New York, NY. 2012.
- Pierru J-F, Girmens E, Héron M, Paques. Occlusions veineuses rétiniennes. Journal Français d’ophtalmologie. 2017; 40: 696-705.
- Prisco D, Marcucci R, Bertini L, Gori AM. Cardiovascular and thrombophilic risk factors for central retinal vein occlusion. Eur J Intern Med. 2002; 13: 163–169.
- Klein R, Moss SE, Meuer SM, Klein BEK. The 15-year cumulative incidence of retinal vein occlu-sion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008; 126: 513–518.
- O’Mahoney PRA, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for athero-sclerosis. Arch Ophthalmol. 2008; 126: 692–699.
- The Central Vein Occlusion Study Group: Natural history and clinical management of central retinal vein occlusion. 1997 Arch Ophthalmol, 115, 486–491.
- M Rehak, P Wiedemann. Retinal vein thrombosis: Pathogenesis and management. Journal of Thrombosis and Hemostasis. 2010; 8: 1886-1894.
- G Coscas, A Loewenstein, A Augustin, F Bandello, M Battaglia Parodi, P Lanzetta, et al. Management of Retinal Vein Occlusion - Consensus Document Ophthalmologica. 2011; 226: 4-28.