
Editorial
Austin J Clin Ophthalmol. 2018; 5(3): 1095.
Phototransduction in a Rabbit Model with Retinitis Pigmentosa
Asakawa K* and Ishikawa H
Department of Orthoptics and Visual Science, Kitasato University, School of Allied Health Sciences, Kanagawa, Japan
*Corresponding author: Ken Asakawa, Department of Orthoptics and Visual Science, Kitasato University, School of Allied Health Sciences, 1-15-1 Kitasato, Sagamihara, Minami-ku, Kanagawa 252-0373, Japan
Received: July 31, 2018; Accepted: August 21, 2018; Published: August 28, 2018
Introduction
Retinitis pigmentosa (RP) is a progressive disease that exhibits abnormalities in the photoreceptor cells of the rods and cones that detect light, and is characterized by symptoms such as night blindness, visual field constriction especially in the peripheral field, and reduced visual acuity [1]. In Japan, this disorder is estimated to affect approximately 20 in 100,000 people and is the third leading cause of blindness following glaucoma and diabetic retinopathy [2]. RP appears through a variety of genetic inheritance modes, including autosomal dominant, autosomal recessive, and X-linked recessive patterns, and 40 or more causative genes have been identified [3]. In many patients, however, genetic abnormalities cause this disorder to be under diagnosed.
To overcome these difficulties, transgenic (Tg) rabbits with a P347L rhodopsin mutation were created by Kondo et al. [4]. They displayed symptoms extremely similar to findings in patients with RP and are considered to offer a useful animal model for elucidating the pathology of RP [5]. However, when and where the degeneration begins are unclear, as are its underlying mechanisms and pathologies. There are many other unknown issues as well, such as how light is received and converted to electric signals in the steps leading to blindness, and why organisms with undeveloped vision such as rabbits receive light, and where photoreceptors are located in these animals. In humans, light is received by photoreceptor cells and is converted to electric signals to visualize an object.
In clinical settings, we encounter patients with RP who maintain circadian rhythms in which the body wakes in the morning and becomes sleepy at night, and those who present a residual light response in which the pupil becomes smaller on exposure to light. This is unlike patients who have lost their vision due to other eye diseases. In other words, RP patients show partial response to light even when blind and unable to sense light. Based on these facts, we hypothesized the existence of photoreceptors other than the rods and cones of the photoreceptor cells. In this review based on our previous studies [6,7], we report the mechanism of retinal degeneration and describe the process of phototransduction in Tg rabbits with a P347L rhodopsin mutation.
Pupil Response
The photoreceptor cells control the constriction and dilation of the pupil in response to change in light stimulus, namely the pupil response. Figure 1 shows (A) the percentage pupil constriction results to red light (635nm) and (B) blue light (470nm) with intensity of 2.3 log cd/m2 for 60s in the Wild-type (WT) and Tg rabbits. The mean values at 4 months was 20.8% for red light and 61.1% for blue light in the WT rabbits and 19.6% for red light and 59.4% for blue light in the Tg rabbits. The differences between the two types were not significant (red, p=0.66; blue, p=0.45, Mann-Whitney U test). At 8 months, the red light was slightly reduced in the Tg rabbits (13.8%). This value was significantly lower than that of the WT rabbits (28.5%; p<0.01). The values for the WT rabbits at 12 months were 25.0% for red light and 56.2% for blue light, whereas the Tg rabbits indicated a loss of response to red light (6.1%); however, pupil constriction to blue light was still induced (20.3%). The values for the Tg rabbits were also significantly smaller than those of the WT rabbits (red and blue, p<0.01). At 24 months, pupil constriction even in blue light stimulus was eliminated (7.9%).
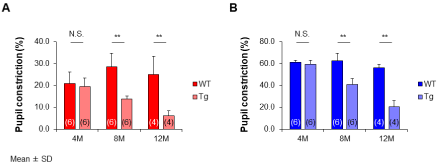
Figure 1: (A) The red and (B) the blue light stimulus. Tg rabbits decreased
with increasing age, and the percentage pupil constriction is significantly
small compared to the age-matched WT rabbits (8 and 12 months: red and
blue, p<0.01) except at 4 months (red, p=0.66; blue, p=0.45). At 12 months,
red light has almost disappeared, while that to blue light is still being induced
in the Tg rabbits. At 24 months, blue light is also eliminated. WT, wild type;
Tg, transgenic; M, months. **p<0.01: Mann-Whitney U test. Data are shown
as mean values ± standard deviation (SD). The numbers in the parentheses
indicate the number of animal eyes used.
Electroretinogram
The electroretinogram (ERG) is a valuable tool for understanding retinal function (a-wave: photoreceptor cells component, b-wave: bipolar cells component) in both human patients and animal models of RP, even in early stage. Figure 2 shows the ERG with a full visual field stimulation at 2.3 log cd s/m2 of emission intensity results of the (A) a-wave and (B) b-wave for the WT and Tg rabbits. At 4 months, there was no significant difference between the Tg and WT rabbits (a-wave, p=0.28; b-wave, p=0.52). The mean amplitude of the a-waves in the WT rabbits at 4 months was 190 μV and of the b-waves, 385μV. These values of WT rabbits at 8 months were 147μV and 339μV. In 12 months, a-waves were 142μV and b-waves were 328μV. Meanwhile, in the Tg rabbits, the a-wave decreased from 174μV at 4 months to 86μV at 8 months, and the b-waves decreased from 360μV to 255μV. At 12 months, the ERG response had considerably decreased for both the a-wave (56μV) and b-wave (221μV). The a-wave and b-wave were significantly lower in the Tg rabbits than in the WT rabbits (8 and 12 months: a-wave and b-wave, p<0.01).
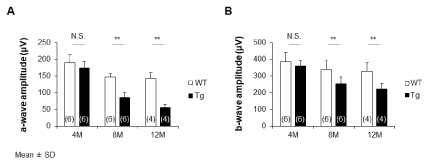
Figure 2: (A) The amplitude of a-waves and (B) b-waves of Tg rabbits at 8
and 12 months is significantly smaller than those of the age-matched WT
rabbits (8 and 12 months: a-wave and b-wave, p<0.01) except for those at 4
months (a-wave, p=0.28; b-wave, p=0.52).
Dissecting Microscopy
The WT fundus has an opaque myelinated portion that extends horizontally from the optic nerve head (Figure 3A). The optic nerve of Tg rabbits at 8 months was characterized by a slight degeneration of the myelin sheath, and the degeneration was characterized by the loss of nerve fiber. Deformation of the myelin sheath became remarkable by 12 months. Optic disc cupping and optic atrophy were observed at 24 months (Figure 3B).
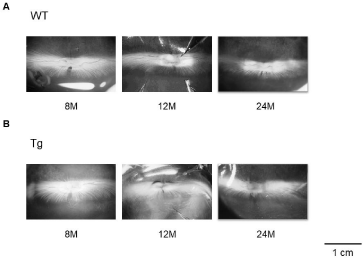
Figure 3: At 8 months, degeneration of the myelin sheath of the optic nerve
has progressed slightly more in Tg rabbits. The loss of nerve fiber and
deformation of the myelin sheaths are remarkable by 12 months. Optic disc
cupping, optic atrophy, and decreasing of other nerve fibers are seen at 24
months.
Light Microscopy
At 8 months, the overall photoreceptor structure appeared almost normal. At 12 months, the whole retina thickness became thinner because of loss of the outer nuclear layer (ONL) and the photoreceptor layer in the peripheral portions, and the rods and cones disappeared. Figure 4A shows the WT rabbits’ optic disc at 24 months. In the Tg rabbits’ optic disc, myelinated axons were sparsely distributed, and growth of astrocytes, i.e., astrocyte infiltration was observed (Figure 4B).
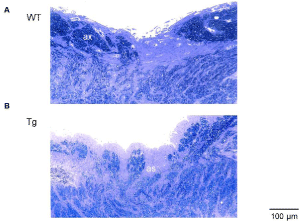
Figure 4: In the optic disc, (A) myelinated axons (ax) of the WT rabbit are
densely crowded. (B) Those of the Tg rabbit are sparsely distributed, and the
astrocyte infiltration (as) are observed.
Electron Microscopy
Comparing the WT rabbits at 24 months (Figure 5A) with the Tg rabbits (Figure 5B), the number of nuclei from the ONL had decreased at 8 months. Simultaneously, the cell pyknosis in the nucleus of the ONL was observed in the visual streak. At 12 months, the retinal cells of the inner nuclear layer were observed to make direct contact with the portion of the retinal pigment epithelium in the peripheral area. The rods had disappeared, and the remaining cones exhibited ballooning degeneration in the central area (Figure 5C). At 24 months, in the periphery of the retina, there is a vacuolar degeneration of retinal ganglion cells (RGCs), and hypertrophy and proliferation of Müller cells (Figure 5D). Degeneration of myelinated axons in the optic disc and damage of the myelin sheath of small axons in the optic nerve were observed (Figure 5E).
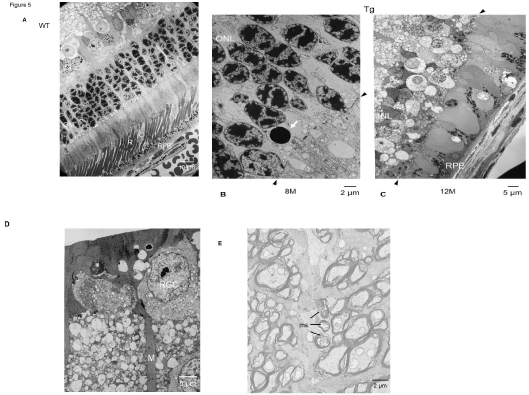
Figure 5: (A) Comparison of the WT rabbits at 24 months, with Tg rabbits; (B) cell pyknosis (white arrow) in the nucleus of the outer nuclear layer (ONL) is observed
in the central area of the visual streak at 8 months. Rods (R) have disappeared, and the remaining cones (C) exhibit ballooning degeneration. (C) The retinal cells
of the inner nuclear layer (INL) are in direct contact with the retinal pigment epithelium (RPE) at 12 months. Two black arrows show the location of the outer limiting
membrane. (D) Vacuolar degeneration of retinal ganglion cell (RGC) and hypertrophic proliferation of Müller cells (M) are observed. (E) Degeneration of myelinated
axons in the optic disc. Damage of the ring-like structure of the myelin sheath (ms) of small axons in the optic nerve.
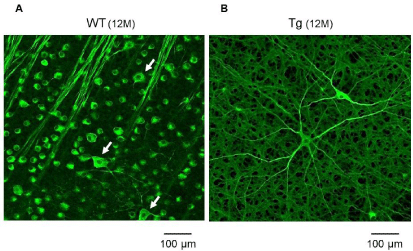
Figure 6: (A) TUJ-1 immunofluorescent staining of the RGCs shows large (white arrows) and small cells in the WT rabbits at 12 months. In the Tg rabbits, the
number of small cells is decreased compared to the WT rabbits. (B) A few remaining RGCs have large cell bodies, which have an extensively branching dendritic
structure with long dendrites, are more clearly observed even in Tg rabbits at 12 months.
Immunofluorescent Staining with Specific Antibody
Alexa Fluor® labeled neuronal class III beta-tubulin (TUJ-1; Molecular Probes, Eugene, OR, USA) 1:500 monoclonal antibodies were used for immunofluorescent staining of the RGCs. Even at 12 months, subtype of the RGCs showed both large and small cells in the WT rabbits (Figure 6A). In the Tg rabbits, the remaining large cells, which have an extensively branching dendritic structure with long dendrites, were more clearly observed, whereas the number of small cells has decreased (Figure 6B).
Discussion
Fundamental treatment methods for RP are currently lacking. To delay progression, patients are sometimes given helenien, vitamin A, and medications that improve circulation, but the effects of these oral therapeutics have yet to be confirmed [8]. In some countries, clinical trials of artificial retina with embedded electrodes are beginning, but concerns remain that transplantation surgery is highly invasive and expensive [9]. While elucidation of the mechanisms of degeneration and pathology of this disorder will not immediately lead to improved diagnoses and treatment modalities, we anticipate that these results will eventually facilitate prevention and the further development of therapies. Moreover, if this study can elucidate the mechanisms of degeneration and the process of phototransduction in a new photoreceptor other than photoreceptor cells, then the results may be applicable to the provision of alternative vision not only for patients with RP but also for patients who have lost their vision due to impairment of photoreceptor cells, such as age-related macular degeneration.
In the present review, the pupil response and the ERG components in the Tg rabbits decreased with increasing age, and differences were found compared to the age-matched WT rabbits. At 12 months of age, pupil constriction to red light had disappeared; however, it was still induced during exposure to blue light.
The pupil response has two main pathways: an afferent and an efferent limb. The afferent pathway leaves the optic tract before the lateral geniculate body and terminates in the midbrain. Therefore, retinal photoreceptor cells as the origin of the pupil response were regarded as the rods and cones for more than 100 years. Why then do rabbits with damage to retinal photoreceptor cells maintain the pupil response to blue light?
Provencio et al. isolated melanopsin, an intrinsically photosensitive visual pigment, from dermal melanophores in Xenopus laevis and reported that melanopsin was present in RGCs of inner retina [10]. These RGCs are known as melanopsin-containing RGCs (mRGCs). mRGCs account for only about 1% of the total number of RGCs, but have large cell bodies and an extensively branching dendritic structure with long dendrites [11]. Berson et al. found that mRGCs have projections to the suprachiasmatic nucleus as master clock of the circadian rhythm [12]. They also project to the olivary pretectal nucleus and Edinger-Westphal nucleus in the midbrain and play a role in the pupil response [13,14]. These photoreceptions by mRGCs have a selective sensitivity to blue light with a wavelength of 460–480 nm [15-17].
Morphologically, light and electron microscopy showed a progressive loss of rods over time from 8 months of age, especially in the peripheral retina. Additionally, pyknoses of the nuclei of the ONL were observed at 8 months. Our images of pyknotic nuclei in the ONL, which most likely represent dying apoptotic photoreceptors, do not adequately explain the underlying mechanism of the disease process that results from the mutant protein expressed in rod photoreceptor cells. Other mechanisms of photoreceptor cell death may be involved, including rhodopsin overproduction, prolonged phototransduction events or mislocalized opsin [18-20]. In the future, these evaluations should be included to confirm this pathogenic event. However, the cell pyknosis suggests that it is an early change associated with retinal photoreceptor cell degeneration in Tg rabbits.
In the late stages of RP, the small type of RGCs and nerve fibers were particularly damaged and hypertrophic proliferation of Müller cells was observed. They subsequently lead to optic disc excavation and atrophy of the optic nerve. The reason was that the bipolar cells and RGCs lost association with the loss of rods and cones because of the degeneration of cells in the periphery of the retina, and those degenerated cells underwent phagocytosis due to Müller cells being subject to hypertrophic growth [21]. We also observed the disruption of the myelin sheath of small axons that occurred with the degeneration of axons and atrophy of the optic nerve. Optic disc cupping could closely be associated with optic atrophy [22]. Therefore, these morphological findings indicated that RP is caused by irreversible degeneration of neural cells. Of note, some remaining RGCs had large cell bodies with long branching dendritic structures as that with the mRGCs, even in a rabbit. Consequently, our findings indicated that the phototransduction of Tg rabbits after complete retinal photoreceptor loss is mediated by mRGCs.
In conclusion, the existence of these residual mRGCs despite advanced degeneration of rods and cones, indicate the fact that mRGCs are involved in synchronization of the circadian rhythm and are primitive photoreceptors in the sleep-wake cycle.
Acknowledgements
We are grateful to Prof. Mineo Kondo of Mie University Graduate School and Prof. Hiroko Terasaki of Nagoya University Graduate School for providing the transgenic rabbits with a P347L rhodopsin mutation, Dr. Hidehiro Oku of Osaka Medical College for the technical assistance in TUJ-1 immunostaining, Mr. Shigeyoshi Maruyama of the Center for Genetic Studies of Integrated Biological Functions and the Research Center for Biological Imaging of Kitasato University for the anesthesia and the electron and confocal laser scanning microscopy. We also thank Mr. Robert E. Brandt of MedEd Japan for editing the manuscript. All the figures were modified and reproduced with permission from references [6] and [7]. This study was supported by a grant from Kitasato University Research Grant for Young Researchers (2018).
References
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006; 368: 1795–1809.
- Hosono K, Minoshima S, Hotta Y. Retinitis pigmentosa in Japanese population. Prakash G, Iwata T, editors. In: Essentials in Ophthalmology: Advances in Vision Research. Springer Press. 2017; 111–128.
- Nakazawa M. New era for molecular diagnosis of retinitis pigmentosa: From research to therapy. Austin J Clin Ophthalmol. 2014; 1: 1019.
- Kondo M, Sakai T, Komeima K, Kurimoto Y, Ueno S, Nishizawa Y, et al. Generation of a transgenic rabbit model of retinal degeneration. Invest Ophthalmol Vis Sci. 2009; 50: 1371–1377.
- Jones BW, Kondo M, Terasaki H, Watt CB, Rapp K, Anderson J, et al. Retinal remodeling in the Tg P347L rabbit, a large-eye model of retinal degeneration. J Comp Neurol. 2011; 519: 2713–2733.
- Asakawa K, Ishikawa H, Uga S, Mashimo K, Shimizu K, Kondo M, et al. Functional and morphological study of retinal photoreceptor cell degeneration in transgenic rabbits with a Pro347Leu rhodopsin mutation. Jpn J Ophthalmol. 2015; 59: 353–363.
- Asakawa K, Ishikawa H, Uga S, Mashimo K, Kondo M, Terasaki H. Histopathological changes of inner retina, optic disc, and optic nerve in rabbit with advanced retinitis pigmentosa. Neuroophthalmology. 2016; 40: 286–291.
- Nakazawa M, Ohguro H, Takeuchi K, Miyagawa Y, Ito T, Metoki T. Effect of nilvadipine on central visual field in retinitis pigmentosa: a 30-month clinical trial. Ophthalmologica. 2011; 225: 120–126.
- Mills JO, Jalil A, Stanga PE. Electronic retinal implants and artificial vision: journey and present. Eye (Lond). 2017; 31: 1383–1398.
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000; 20: 600–605.
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, et al. Melanopsin-expressing ganglion cells in primate retina signal color and irradiance and project to the LGN. Nature. 2005; 433: 749–754.
- Berson DM. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003; 26: 314–320.
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001; 4: 621–626.
- Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003; 23: 7093–7106.
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007; 47: 946–954.
- Ishikawa H. Pupil and melanopsin photoreception. Nippon Ganka Gakkai Zasshi. 2013; 117: 246–268.
- Asakawa K, Ishikawa H. Why do melanopsin-containing retinal ganglion cells have the greatest sensitivity to blue light? Acta Ophthalmol. 2015; 93: 308–309.
- Tan E, Wang Q, Quiambao AB, Xu X, Qtaishat NM, Peachey NS, et al. The relationship between opsin overexpression and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2001; 42: 589–600.
- Chen J, Makino CL, Peachey NS, Baylor DA, Simon MI. Mechanisms of rhodopsin inactivation in vivo as revealed by a COOH-terminal truncation mutant. Science. 1995; 267: 374–377.
- Alfinito PD, Townes-Anderson E. Activation of mislocalized opsin kills rod cells: a novel mechanism for rod cell death in retinal disease. Proc Natl Acad Sci USA. 2002; 99: 5655–5660.
- Szamier RB, Berson EL. Histopathologic study of an unusual form of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1982; 22: 559–570.
- Lahav M, Craft J, Albert DM, Ishii Y. Advanced pigmentary retinal degeneration: an ultrastructural study. Retina. 1982; 2: 65–75.