Review Article
Austin J Clin Pathol. 2014;1(1): 1001.
Role of Stem Cell-derived Extracellular RNA-carrying Vesicles in Cell Reprogramming
Giovanni Camussi1,*, Maria Chiara Deregibus1 and Peter J Quesenberry2
1Department Medical Sciences, University of Torino, Italy
2Department of Medicine, the Warren Alpert Medical School of Brown University, USA
*Corresponding author: Giovanni Camussi, Department Medical Sciences, University of Torino, Italy
Received: January 27, 2014; Accepted: March 17, 2014; Published: March 20, 2014
Abstract
There is increasing evidence indicating that secreted RNA molecules may act as paracrine/endocrine mediators, capable of modifying the phenotype of target cells. Several studies have focused on transporters of extracellular RNAs, indicating that they may be present in biological fluids in a non-encapsulated form, or encapsulated within membrane vesicles. Cell-derived extracellular vesicles, including exosomes and microvesicles, have recently emerged as a well-preserved evolutionary mechanism of cell-to-cell communication. It has been shown that extracellular vesicles can contain several species of RNAs, including mRNAs, microRNAs and long non-coding RNAs. Vesicleencapsulated extracellular RNAs are protected from degrading enzymes, and can be delivered after internalization or membrane fusion to target cells. The transfer of nucleic acids may induce epigenetic changes in the recipient cells with functional consequences. Here we discuss the role of extracellular vesicles in the cross talk between stem and injured cells, and in the resulting reprogramming of these cells.
Introduction
Cell differentiation and cell phenotypes are critically modulated in a defined microenvironment by exchange of information between cells. Emerging evidence indicates that, in addition to cytokines, chemokines and hormones [1], secreted RNA molecules [2] may act as endocrine/paracrine mediators. Extracellular RNAs (exRNAs) have been identified in all human biological fluids, either in a vesicleencapsulatedform, or as nuclease-resistant complexes with RNA binding proteins such as high-density and low-density lipoproteins and Argonaut proteins.
Vesicle-encapsulated exRNAs are protected from RNAdegrading enzymes and can be delivered locally or at distant sites as a consequence of vesicle uptake by target cells. Cell-secreted vesicles express membrane receptors of donor cells, and may interact with cells bearing counterpart receptors or through surface-expressedlipids. As a result of this interaction, vesicles are internalized into endocytic compartments or become directly fused with the plasma membrane, thus transferring their bioactive contents into recipient cells, inducing epigenetic changes of their phenotype.
The release of pre-apoptotic vesicles and apoptotic bodies is known by long time. However, the role of vesicles released by healthy cells in the intercellular communication has only recently emerged.
Non-apoptotic vesicles have been named in the literature on the basis of function, biogenesis or cell of origin as microparticles, prostatosomes, cardiosomes, tolerosomes, ectosomes, microvesicles and exosomes [3-5].
The term of exosomes is usually reserved to vesicles derived from the endosomal membrane compartment by exocytosis, whereas microvesicles are used to design vesicles generated by budding of cell plasma membranes [6, 7]. Given the differences in biogenesis and composition of vesicles derived from different cellular sources, as well as the presence of overlapping characteristics between exosomes and microvesicles, the use of the inclusive term of "extracellular vesicles" (EVs) has been suggested. Vesiculation, which occurs either within the cell or on the cell surface, allows accumulation of transmembrane proteins, cytosolic proteins and nucleic acids, all specific to the cell of origin [4]. Therefore, EVs are potentially capable of different biological activities according to their cargo [5]. Transfer of bioactive lipids, proteins, receptors, mRNA, microRNA (miRNA) and long non-coding RNA (lncRNA) may change the phenotype and function of the recipient cells [6-14]. In particular, the exchange of miRNAs, which regulate most protein-encoding genes, and of lncRNAs, which modulate the epigenome, may influence several physiological and pathological processes. Therefore, the discovery of EV-mediated intercellular communication has revealed an unpredicted plasticity of the cellular system. In the context of stem cell biology, the exchange f genetic information may explain mechanisms involved in the aintenance of stemness or differentiation, as well as in stem cellmediated tissue repair after injury.
Extracellular Vesicles as Transporters of Exrnas
The discovery that EVs contain nucleic acids provides an explanation for the proposed role of exRNA in cell-to-cell communication [2]. In fact, vesicle-encapsulated RNA is protected from extracellular RNase, making EVs an attractive vehicle for the intercellular exchange of RNA. Pivotal studies by Ratajczak et al. [15] showed that murine embryonic stem cells release EVs containing stem cell-specific mRNA. Similarly, tumor-derived EVs were shown by Baj-Krzyworzeka et al. [16] to contain growth factor mRNAs and to transfer them to monocytes. The mRNA present in EVs was shown to be functional when incorporated into recipient cells. Indeed, Valadi et al. [17] provided evidence that EV-transferred mRNA can be translated into proteins, and we also found that, following uptake of EVs carrying GFP mRNA, endothelial cells start to produce the FP protein [18]. The inter-species exchange of RNA, via EVs,further confirmed the functionality of the transferred RNA. Valadiet al. [17] showed that RNA carried by mouse mast cell exosomes was transferable in vitro to human mast cells, resulting in production of mouse proteins in the human mast cells. Moreover, human stem cell-derived EVs were shown to transfer mRNAs into mouse cells, which were subsequently translated into proteins not only in vitro but also in vivo [19,120]. Aliotta et al. [21] demonstrated that lung mRNA was transferred by EVs to bone marrow cells, inducing expression of lung specific proteins. Other RNA species were also shown to be present within EVs, namely miRNA and lncRNA. Valadi et al. showed that mouse and human mast cell exosomes contain miRNAs [17], which can be transferred altering gene expression in recipient cells. Yuan et al. [22] provided evidence for the transfer of miRNAs from mouse embryonic stem cells to fibroblasts. The miRNA content of EVs reflects that of the cell of origin; however, we found an enrichment of selected miRNAs within EVs that were released by human mesenchymal stem cells, suggesting an active process of RNA compartmentalization [23]. The presence of RNA-binding proteins within EVs may shed some light on the loading mechanism of RNA within exosomes/microvesicles [24]. For example, EVs derived from human mesenchymal stem cells (MSCs) were shown to carry Stau 1 and 2, TIA, TIAR and HuR ribonucleoproteins, which are involved in the stability and trafficking of RNA between the nucleus and cytoplasm, as well as proteins of the Argonaute family involved in miRNA transport and processing [23]. In addition, Ago2 and GW182 were found in exosomes that were released from monocytes [24]. Lipids and, in particular, ceramide have been also implicated in the selective accumulation of miRNAs inside EVs [25, 26]. Knock-down of neutral nSMase2, involved in the synthesis of ceramide, was shown to decrease vesicular release of miR-16 and miR-146a [27]. Instead, chemical inhibition of nSMase2 tended to favor the release of miRNA in its non-encapsulated form, in association with HDL [25]
The question that still remains is "are the EV-transferred miRNAs functional?" In response, we found that some target proteins of miRNA carried by stem cell-derived EVs were down-regulated in recipient cells [23]. In addition, Zhang et al. [28] demonstrated that miR-150 delivered by EVs to endothelial cells induced functional alterations and modulated gene expression, including c-Myb expression
EV-Transferred Exrnas Induce Changes in the Cellular Phenotype: Role in Stem Cell Biology
The concept that EV-encapsulated exRNAs may modulate the phenotype and function of target cells is a new paradigm in the interplay between cells. Increasing evidence has indicated that this mechanism of cell phenotype modulation has profound physiological and pathological implications.
In the immune system, the exchange of miRNAs via exosomes between antigen-presenting cells (APC) and T cells has been recently shown to occur at the site of immune synapse [29,30], suggesting a critical role in the initiation and modulation of the immune response [31]. Extensive studies on the role of EVs in the transfer of genetic information have also been performed in the field of tumor biology. Tumor-derived EVs were found to strongly modify the cancer microenvironment, by acting on the phenotype of stromal cells, and by supporting tumor metastases and escape from immune surveillance [32]. Katakowski et al demonstrated that miR-146b carrying exosomes released by bone marrow stromal cells inhibit the growth of glioma xenograft in rats [33]. Microvesicle-mediated transfer of miR-233 was also found to be functionally active as it was able to induce macrophage differentiation [9]. Exosomal miRNAs were also shown to play an important role in communication between leukemia and endothelial cells [10]. Takahashi et al demonstrated that EVs transfer long non-coding RNAs which modulate hypoxia signaling pathways by reprogramming target cells [13].
The role of EV-transferred exRNA in stem cell biology was first addressed by Ratajczak et al. [15] who demonstrated that EVs were implicated in the preservation of pluripotency and in the undifferentiated propagation of stem cells in vitro. EVs released from murine embryonic stem cells (ESC) were shown to be highly enriched in mRNA for several pluripotent transcription factors and to express Wnt-3 protein. Once delivered to target cells, the transferred mRNA was translated into proteins, thus facilitating reprogramming of murine hematopoietic progenitors, enhancing their survival and expansion [15]. Dependency on horizontal transfer of mRNA was suggested by experiments based on RNase inactivation of EVshuttled RNA, which abrogated the biological effects. EV-mediated horizontal transfer of mRNA from endothelial progenitor cells (EPC) also accounted for reprogramming quiescent endothelial cells toward a pro-angiogenic phenotype [18]. We found that EVs secreted from EPC carry specific subsets of pro-angiogenic mRNA including those associated with PI3K/AKT and eNOS [18].
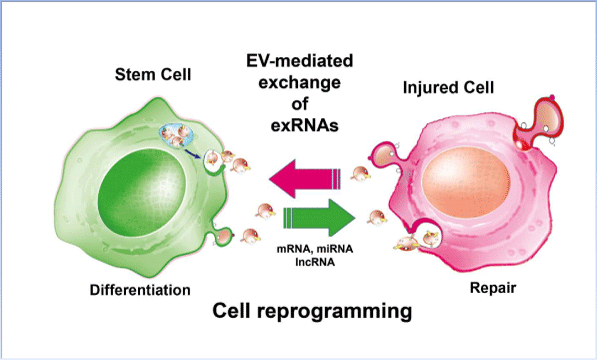
The functional role of EVs was demonstrated by the group of Quesenberry who focused on the ability of EVs to modify the phenotype of bone marrow cells by transfer of nucleic acids and proteins. In addition, Aliotta et al. [21] showed that EVs are able to transfer RNA from injured lung cells to bone marrow cells, inducing lung-specific gene expression such as surfactant B, and surfactant C and Clara cell-specific protein. Their work indicated that cell fate alterations were mediated by a RNA species acting as a transcription factor to induce long-term epigenetic phenotype changes. EVmediated reprogramming may provide an alternative explanation to transdifferentiation or fusion for the bone marrow stem cell plasticity observed during physiologic tissue repair. Based on these observations, Quesenberry and Aliotta suggested that information exchange, mediated by EVs, constitutes an integral component of the continuum model of stem cell biology, and contributes to the repair of injured tissue [34]. Thus, EVs may allow a mutual influence between stem and injured cells based on bidirectional transfer of information that results in functional and phenothipic changes. EVs released from somatic cells may influence stem cell plasticity; conversely, those released from stem cells may activate regenerative programs in cells survived to injury (Figure 1). We have studied this possibility in the kidney, where it is known that the repair following acute tubular injury mainly depends on the de-differentiation of tubular cells to a mesenchymal phenotype, with re-entry into the cell cycle of cells that have survived injury [35]. The beneficial effect of MSC-based therapy in acute kidney injury (AKI) models has been ascribed to paracrine mechanisms rather than trans-differentiation or fusion [36]. Therefore, in this context, EVs released from stem cells are potential candidates for reprogramming injured cells and for coordination of repair. Indeed, we have found that EVs from human MSCs can mimic the biological effects of the cells of origin, promoting functional and morphological repair of glycerol-induced AKI [19]. After EV incorporation, renal tubular epithelial cells dedifferentiated to a stem cell-like phenotype, acquired apoptosis resistance, proliferated, in order to facilitate tubule repopulation,and finally re-differentiated into mature epithelial cells. MVs derived from MSCs were demonstrated to contain several mRNAs that were specific to the mesenchymal lineage, as well as mRNAs involved in the control of transcription, proliferation, cell fate and immune regulation [19]. The in vivo transfer of human mRNA to murine tubular cells and its transient translation into protein was observed [19]. Moreover, in a lethal model of cisplatin-induced AKI in SCID mice, EVs derived from MSCs were shown to protect mice from death, and significantly improved renal function by inducing up-regulation of the anti-apoptotic genes, BIRC8, Bcl2 and Bcl-xL, and downregulation of genes involved in the execution-phase of apoptosis, namely LTA, Casp1 and Casp8 [37]. Other studies in models of heart ischemia/reperfusion injury [38] and of 70% hepatectomy [20] also support the concept that RNA-mediated changes in cell phenotype after treatment with stem cell-derived EVs favor tissue regeneration.
Figure 1: Schematic representation of EV-mediated bidirectional exchange of exRNAs between stem and injured cells. The transfer of genetic information from injured cells may induce differentiation of stem cells and expression of tissue specific phenotype. Conversely, stem cell-derived exRNAs may activate regenerative programs in recipient cells with activation of mechanisms of tissue self-repair.
The exRNAs involved in cell reprogramming may also include miRNAs [39]. We screened the miRNAs present in MSC-derived EVs, and gene ontology analysis of their targets revealed a high expression of miRNAs implicated in cell differentiation and survival and in immune system regulation [23]. The EV-mediated transfer of miRNA to renal tubular cells was found to down-regulate some target proteins, suggesting functionality of transferred miRNAs. The relevance of miRNA transfer by EVs was particularly evident in a model of ischemia-reperfusion AKI treated with EVs released by EPC that are enriched in angiomiR miR-296 and miR-126 [40]. In this model, when miRNA-depleted EVs obtained by Dicer knockdown EPCs were used, the beneficial effect of EVs on AKI recovery was significantly reduced. Similarly, EV depletion of the specific proangiogenic miRNAs, as well as the use of antagomiRs, decreased the effectiveness of EVs [41].
Recently, the Quesenberry group demonstrated that EVs released by lungs [42] and prostate cancer cells [43] may modify the normal cell genetic profile, by conveying specific subsets of mRNA. Conversely, exRNA derived from normal cells or from stem cells may reprogram tumor cells to a more benign phenotype. Indeed, EVs derived from normal prostate cells were shown to reprogram prostate cancer cells, reversing their intrinsic chemoresistance and their anchorage-independent growth [44], and EVs derived from human liver stem cells were shown to inhibit growth and survival of HepG2 hepatoma and primary hepatocellular carcinoma cells by delivering tumor suppressor miRNAs [45].
Xin et al. showed a crosstalk between MSCs and brain parenchymal cells, with transfer of miR-133b via EVs, and subsequent regulation ofneurite outgrowth [46]. In addition, MSCs transfected with synthetic miR-133b were able to transfer this miRNA, via EVs, to astrocytes and neurons, regulating the gene expression, and favoring neural plasticity and functional recovery after stroke [47]. Yu et al. [48] have recently demonstrated that cardiomyocyte protection may be mediated, at least in part, by the transfer of the anti-apoptotic miR 221/222, via EVs released from GATA-4 overexpressing MSCs. miR- 221/222 is known to decrease the expression of the pro-apoptotic gene p53 upregulated modulator of apoptosis (PUMA). Recently, it has been shown that EVs derived from embryonic stem cells may induce gene expression changes in Müller cells of retina by transfer of mRNA and miRNA (49). Taken together, these experiments indicate that transfer of genetic material from stem cells to somatic cells may change their phenotype and function suggesting a possible exploitation for gene therapy [50].
Conclusions
Investigation into the biological relevance of exRNA-mediated signaling sheds a new light on several physiological and pathological processes, and opens new therapeutic perspectives. EVs emerge as an important, but not unique, transporter of exRNA. EVs may influence the phenotype of neighboring cells, by delivering their content locally, and by entering the circulation, or other biological fluids, they are also able to function at distant sites. The transfer of geneticinformation between stem and injured cells provides a new vision of cell plasticity. EVs released by stem cells retain several biological roles of the cells of origin, and activate regenerative programs in injured cells. However, further studies are necessary before stem cellinduced EVs can effectively become candidates for use in therapy. In particular, healing exRNAs that are transported by EVs have yet to be identified. A better understanding of the mechanisms involved in exRNA compartmentalization within EVs may provide information, allowing production of engineered vesicles containing specific subsets of RNA. Moreover, as EVs are a non-homogenous population, it is essential to know which of the EV fractions contain curative exRNA, starting from the characterization of the biologically active population. Finally, the scalable production of EVs under Good Manufacturing Practice (GMP) conditions is a particular challenge. This nascent research field is promising, not only for a better characterization of pathogenic mechanisms, but also for the identification of diagnostic/prognostic biomarkers and the development of new therapeutic approaches.
Acknowledgements
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award number UH2TR000880. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Peinado H1, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011; 21: 139-146.
- Stroun M, Anker P, Beljanski M, Henri J, Lederrey C, Ojha M, et al. Presence of RNA in the nucleoprotein complex spontaneously released by human lymphocytes and frog auricles in culture. Cancer Res. 1978; 38: 3546-3554.
- Cocucci E1, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009; 19: 43-51.
- György B1, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011; 68: 2667-2688.
- György B1, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011; 68: 2667-2688.
- Raposo G1, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200: 373-383.
- Akers JC1, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013; 113: 1-11.
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. (1999) Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999; 94: 3791-3799.
- Lee TH1, D'Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer--the emerging science of cellular 'debris'. Semin Immunopathol. 2011; 33: 455-467.
- Ismail N1, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K, et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013; 121: 984-995.
- Umezu T1, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013; 32: 2747-2755.
- Al-Nedawi K1, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009; 8: 2014-2018.
- Ratajczak J1, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006; 20: 1487-1495.
- Takahashi K, Yan IK, Haga H, Patel T. Modulation of hypoxia-signaling pathways by extracellular long non-coding RNA regulator of reprogramming. J Cell Sci. 2014; .
- Wysoczynski M1, Ratajczak MZ. Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int J Cancer. 2009; 125: 1595-1603.
- Ratajczak J1, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006; 20: 847-856.
- Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, et al. (2006) Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006; 55: 808-818.
- Valadi H1, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007; 9: 654-659.
- Deregibus MC1, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007; 110: 2440-2448.
- Bruno S1, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009; 20: 1053-1067.
- Herrera MB1, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010; 14: 1605-1618.
- Aliotta JM1, Pereira M, Johnson KW, de Paz N, Dooner MS, Puente N, et al. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp Hematol. 2010; 38: 233-245.
- Yuan A1, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, et al. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS One. 2009; 4: e4722.
- Collino F1, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010; 5: e11803.
- Gibbings DJ1, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009; 11: 1143-1149.
- Vickers KC1, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol. 2012; 23: 91-97.
- Trajkovic K1, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008; 319: 1244-1247.
- Kosaka N1, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010; 285: 17442-17452.
- Zhang Y1, Liu D, Chen X, Li J, Li L, Bian Z, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010; 39: 133-144.
- O'Connell RM1, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010; 10: 111-122.
- Mittelbrunn M1, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MÁ, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011; 2: 282.
- Bobrie A1, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011; 12: 1659-1668.
- Castellana D1, Kunzelmann C, Freyssinet JM. Pathophysiologic significance of procoagulant microvesicles in cancer disease and progression. Hamostaseologie. 2009; 29: 51-57.
- Katakowski M1, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013; 335: 201-204.
- Quesenberry PJ1, Dooner MS, Aliotta JM. Stem cell plasticity revisited: the continuum marrow model and phenotypic changes mediated by microvesicles. Exp Hematol. 2010; 38: 581-592.
- Humphreys BD1, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008; 2: 284-291.
- Otto WR1, Wright NA. Mesenchymal stem cells: from experiment to clinic. Fibrogenesis Tissue Repair. 2011; 4: 20.
- Bruno S1, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012; 7: e33115.
- Timmers L1, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AA, et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011; 6: 206-214.
- Kuo CH1, Ying SY. MicroRNA-mediated somatic cell reprogramming. J Cell Biochem. 2013; 114: 275-281.
- Gatti S1, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011; 26: 1474-1483.
- Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012; 82: 412-427.
- Del Tatto M1, Ng T, Aliotta JM, Colvin GA, Dooner MS, Berz D, et al. Marrow cell genetic phenotype change induced by human lung cancer cells. Exp Hematol. 2011; 39: 1072-1080.
- Renzulli JF 2nd1, Del Tatto M, Dooner G, Aliotta J, Goldstein L, Dooner M, et al. Microvesicle induction of prostate specific gene expression in normal human bone marrow cells. J Urol. 2010; 184: 2165-2171.
- Panagopoulos K1, Cross-Knorr S, Dillard C, Pantazatos D, Del Tatto M, Mills D, et al. Reversal of chemosensitivity and induction of cell malignancy of a non-malignant prostate cancer cell line upon extracellular vesicle exposure. Mol Cancer. 2013; 12: 118.
- Fonsato V1, Collino F, Herrera MB, Cavallari C, Deregibus MC, Cisterna B, et al. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells. 2012; 30: 1985-1998.
- Xin H1, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012; 30: 1556-1564.
- Yu YM1, Gibbs KM, Davila J, Campbell N, Sung S, Todorova TI, et al. MicroRNA miR-133b is essential for functional recovery after spinal cord injury in adult zebrafish. Eur J Neurosci. 2011; 33: 1587-1597.
- Yu B1, Gong M, Wang Y, Millard RW, Pasha Z, Yang Y, et al. Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS One. 2013; 8: e73304.
- Katsman D1, Stackpole EJ, Domin DR, Farber DB. Embryonic stem cell-derived microvesicles induce gene expression changes in Müller cells of the retina. PLoS One. 2012; 7: e50417.
- Y1, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012; 21: R125-134.