Review Article
Austin J Clin Pathol. 2014;1(1): 1003.
Monoclonality and Immunophenotype of Intraepithelial Lymphocytes in Lymphocytic Gastritis
M Rodriguez-Justo1*,Z Avila1, I Proctor1, D Thind1, T Diss1, C Santonja2
1Department of Histopathology, University College London, University Street, London WC1E 6JJ, UK. Tel: (+44) (0) 20 7679 6015;
2Department of Anatomic Pathology, Fundacion Jimenez Diaz, Madrid, Spain;
*Corresponding author: M Rodriguez-Justo, Department of Histopathology, University College London, University Street, London WC1E 6JJ, UK. Tel: (+44) (0) 20 7679 6015; USA
Received: February 09, 2014; Accepted: March 11, 2014; Published: March 22, 2014
Abstract
Aims: To investigate the clonality and immunophenotype of intraepithelial lymphocytes (IELs) in lymphocytic gastritis (LG).
Methods and Results: The study included 47 cases of LG. Clinical information regarding Helicobacter pylori (Hp) infection and coeliac disease (CD) were recorded. Immunohistochemistry for Hp, CD3, CD4 and CD8 (single and double-staining with CD3); and PCR amplification of the T-cell receptor gamma (TCR-?) gene was performed in each case. 11/47 cases were positive for Hp and 2/47 cases had CD. In all cases the IELs showed a CD3+ CD4- CD8+ immunophenotype. DNA quality allowed PCR analysis in 34/47 cases: in 5/34 a monoclonal expansion of T-cells was demonstrated. Three cases were associated with Hp and the remaining cases had no evidence of Hp or CD. None of the patients have developed lymphoma (median follow up 64 months).
Conclusions: Hp infection may trigger LG and induce infiltration of T-lymphocytes into the gastric mucosa. In a significant proportion of cases (15% overall and 43% of Hp+ cases in our series) a T-cell clonal expansion may develop. Some patients with monoclonal T-cell expansion are not Hprelated and other host-related factors may play a role. Although clonal LG, when isolated, appear to be a monoclonal benign disease, there is still need to characterize LG on an immunophenotypical and molecular clonal/level in larger scale follow-up studies. TCR gene rearrangement testing in the diagnosis of a histologically gastric biopsy that only shows features of LG, might contribute to identify patients which may require close follow-up.
Keywords: Gastritis; Immunophenotyping; T-cell gene rearrangement; Helicobacter pylori; Celiac disease; Etiology; Gastric lymphoma
Abbreviations
IELs: Intraepithelial Lymphocytes; LG: Lymphocytic Gastritis; Hp: Helicobacter pylori; CD: Celiac Disease; TCR-?: T-cell receptor gamma; EATL: Enteropathy-associated T-cell lymphoma; H&E: Haematoxylin and Eosin; PCR: Polymerase Chain Reaction.
Introduction
Lymphocytic gastritis (LG) is an uncommon histopathological subtype of chronic gastritis. It is characterised by a marked increase in the number of intraepithelial lymphocytes (IELs) in the gastric surface and foveolar epithelium, together with a variable increase in lymphocytes within the lamina propria [1]. IELs in LG are primarily CD3+/CD8+ cytotoxic T-cells [2,3] and 25 or more IELs per 100 gastric epithelial cells are usually considered diagnostic [4]. The disorder may affect the whole stomach and the reported prevalence varies from 0.83% of unselected patients undergoing endoscopy, to 1.6-4.5% of patients with histological chronic active gastritis [5,6] and 1-8% of patients with dyspepsia [7].
The pathogenesis of LG is poorly understood and its etiology is unknown in a high proportion of cases. It is associated with endoscopic findings of varioliform gastritis [8] (prominent mucosal folds in the gastric fundus with nodules and chronic superficial erosions) although some cases have normal endoscopic appearances.Helicobacter pylori (Hp) infection has been detected histologically or serologically in 40-80% of cases of LG in some studies and it has been suggested that LG may represent an atypical immune response to a local antigen such as Hp [9]. In addition, LG has been associated with gastric adenocarcinoma (prevalence 12%) and primary gastric lymphoma (prevalence 14%-32%) [10,11] two conditions also linked with Hp infection.
Gluten is another suspected inciting agent in some cases of LG. Studies have shown that 36-45% of cases of patients with coeliac disease (CD) may have LG [12-14]. The intraepithelial lymphocytosis in the small bowel in CD has been the focus of much attention. Ulcerative jejunitis and refractory CD are complications and characterised by loss of response to a gluten-free diet and the presence of a cytologically normal, non-invasive, monoclonal intraepithelial T-cell population. Detection of this clonal intraepithelial T-cell population is associated with poor outcome and higher risk of developing enteropathyassociated T-cell lymphoma (EATL) [15]. Bagdi et al [l6] showed that the monoclonal T cell-populations observed in ulcerative jejunitis and refractory CD share an identical aberrant immunophenotype with the monoclonal T-cell population in the lymphoma and adjacent enteropathic mucosa in EATL. Thus the intrepithelial lymphocytosis in refractory CD and ulcerative jejunitis is considered a neoplastic T-cell disorder. In addition, refractory CD is recognised as a diffuse gastrointestinal disease: one study detected a monoclonal T-cellpopulation in 62%, 80% and 44% of gastric, colonic and blood samples respectively from patients with refractory CD [17].
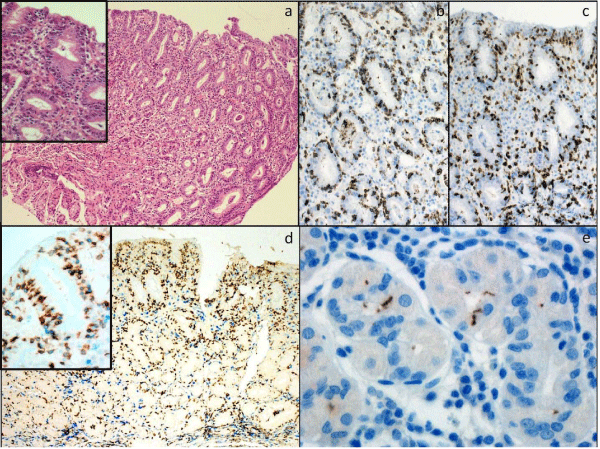
Figure 1: Gastric antral biopsies showing lymphocytic gastritis. A: There is a diffuse lymphocytosis of the surface and foveolar epithelium (H&E x200, inset H&Ex400) [>25 small mature IEL are present per 100 epithelial cells]. B: CD3 (x400), C: CD8 (x400); D: CD3(blue)/CD8(brown) (x200, x600 inset); E: Helicobacter pylori (X600).
The clonality of IELs and potential risk of developing T-cell lymphoma in LG which is not associated with refractory CD has yet to be investigated. In this study we compiled a series of 47 patients diagnosed with LG and studied the immunophenotype and clonality of IELs in this condition. Cases were followed for a median of 64 months. We compared the frequency of monoclonal IELs with respect to clinical and histological information regarding disease association, including Hp infection and CD.
Materials and Methods
Patients
A series of cases which had been reported to show lymphocytic gastritis were selected from the diagnostic archives of the Histopathology Departments at University College London Hospital (London, UK) and Fundacion Jimenez Diaz (Madrid, Spain) between 2000 and 2012 after approval by the institutional Research Ethics Committee (UCL/UCLH Committee Alpha, Reference 09/ H0715/64). Clinical information and serology for Hp infection and CD were recorded in all cases. Duodenal biopsies were available in15 patients and colonic biopsies in 5 patients. Clinical follow up of patients, including development of lymphoma, was recorded for a median of 64 months (range: 15-130 months).
Histology and Immunohistochemistry
All tissue specimens were routinely stained with haematoxylin and eosin (H&E) and examined to confirm the reported histological diagnosis of LG. LG was defined as the presence of 25 or more IELs per 100 gastric columnar epithelial cells (surface and foveolar). [1,6,8]
Immunohistochemical staining was performed on 4 μM sections taken from paraffin wax embedded tissue sections. Staining was performed using Bond III automated immunostainers (Leica). Readyto- use primary antibodies were applied undiluted for CD3 (PA0553), CD4 (PA0368) and CD8 (PA0183) from Leica Microsystems. Double staining with CD3 (blue) and CD8 (brown) was also performed. Antibodies against Hp antibody were applied at a dilution of 1/100 (Dako, UK).
Heat-mediated antigen retrieval (100° C) was used at pH9.0 for 20 min for CD3 and CD8; pH 9.0 for 30 min for CD8; and pH 6.0 for 20 min for Hp. Bond Polymer Refine detection (DS9800) was used with the standard Leica protocol, with an added DAB enhancer step.Incubation without primary antibody was used as a negative control and normal human tonsil was used as a positive control.
Pcr Amplification of The T-Cell Receptor Gamma Chain Gene
DNA was extracted from 10 μM paraffin sections using a commercial kit (Qiagen FFPE DNA extraction kit, UK) following the manufacturer's protocol. Duplicate aliquots of each sample were analysed for rearrangement of the TCR-? chain (tubes A and B) using a standardised BIOMED-2 PCR protocol [18] Positive (T-cell lymphoma) and negative (no template) controls were run in all experiments. Heteroduplex analysis of PCR products was performed using 6% polyacrylamide gels stained with ethidium bromide, and viewed under UV light. This standardised assay has a sensitivity of approximately 5% for detecting a monoclonal cell population in a polyclonal background.
Results
Patients
Forty seven patients with a confirmed histological diagnosis of LG were identified. Review of H&E stained sections confirmed the diagnosis of LG in all cases (Figure 1). The specimens and clinical features are summarised in (Table 1). There was a slight high incidencein males compared with females (57% vs 43%). Median age at diagnosis was 48 years. 11/47 (23%) cases had Hp infection (serology and/or immunohistochemistry); 2/47 (4.3%) had a diagnosis of CD (positive serology and histology); 4/47 (8.5%) had gastric or colonic adenocarcinoma; and 1/47 (2.1%) had a gastric tubular adenoma. Of the 5 patients that had a colonic biopsy available for review, 1/5 (20%) showed lymphocytic colitis. Of the 15 patients that had a duodenal biopsy available for review, 4/15 (27%) showed an increase in IELs. 29/47 (62%) of cases of LG had no known disease association.
Specimens & Clinical features
Number of cases (%)
Gastric specimens:
Biopsy
Gastrectomy
42/47 (89.4%)
5/47 (10.6%)
Gastric biopsy sites:
Antrum
Body
Fundus
Multiple
Unspecified
10/42 (26%)
2/42 (5%)
1/42 (2%)
7/42 (16%)
22/42 (51%)
Sex:
Male
Female
27/47 (57%)
20/47 (43%)
Median age at diagnosis
48y (16-74y)
Associated disease:
Hp positive
CD diagnosis (serology and histology)
Gastric adenocarcinoma
Gastric adenoma
Colonic adenocarcinoma
Nil known
11/47 (24%)
2/47 (4%)
2/47 (4%)
1/47 (2%)
2/47 (4%)
29/47 (62%)
Colonic biopsy available for review:
Lymphocytic colitis
5/47 (11%)
1/5 (20%)
Duodenal biopsy available for review:
Increased IELs
15/47 (32%)
4/15 (27%)
Median follow up
64 months
Developed lymphoma
0/38
Table 1: Specimens and clinical features in 47 patients with a histological diagnosis of lymphocytic gastritis.
Immunohistochemistry
In all 47 cases, immunohistochemistry demonstrated that the majority (>95%) of IELs were CD3+ CD4- CD8+ (Figure 1). The lamina propria lymphocytes showed a mixed pattern of staining (CD3+ CD4- CD8+ or CD3+ CD4+ CD8-).
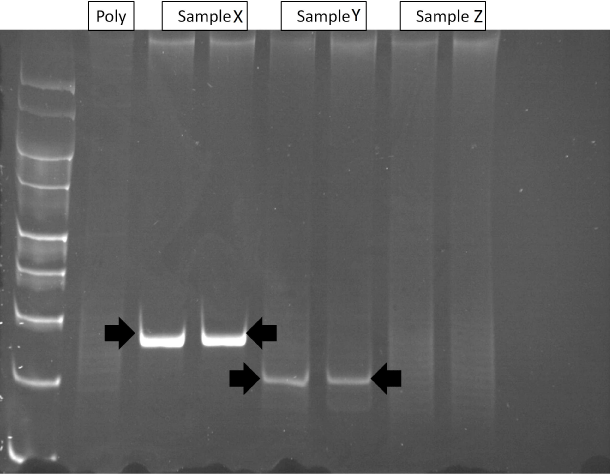
Pcr of Tcr-G Gene In 34/47 cases the extracted DNA was of sufficient quality to allow accurate PCR analysis of the TCR-? and TCR-? gene (Table 2). 5/34 (15%) cases showed a reproducible dominant band indicative of a monoclonal population, whilst 29/34 (85%) showed a 'smear' of different molecular weight amplification products lacking a dominant band indicative of polyclonal populations (Figure 2). TCR-? geneanalysis of Hp positive cases showed a monoclonal band in 43% (3/7). Samples from the two patients with CD and all three patients with adenocarcinoma showed polyclonal populations only. PCR using the BIOMED protocol avoids over-interpretation of oligo- and monoclonality but to exclude the possibility of detecting oligoclonal populations from the T-cells in the lamina propria, microdissection of the epithelial component was also performed in the 5 clonal cases and micro-dissected fractions were run side by side with the whole sections and the same results were obtained.
Disease association & Number of cases
Monoclonal TCR-γ (%)
Polyclonal TCR-γ (%)
Hp infection (n=6)
3/7 (43%)
4/7 (57%)
CD (n=2)
0/2 (0%)
2/2 (100%)
Adenocarcinoma (n=3)
0/3 (0%)
3/3 (100%)
No known association (n=14)
2/22 (9%)
20/22 (91%)
Total (n=25)
5/34 (15%)
29/34 (85%)
Table 2: PCR results for TCR-γ gene rearrangement in 34 cases of lymphocytic gastritis.
Discussion
Forty seven cases of LG were identified in our diagnostic archive between 2000 and 2012 reflecting the low prevalence of this condition. All cases showed an increased number of CD3+ CD4-CD8+ IELs, in keeping with previous studies showing a cytotoxic T-cell phenotype of lymphocytes in LG. The majority of LG cases(62%) had no recognised disease association, although 24% of cases had evidence of Hp infection, and a few cases were associated with gastric adenocarcinoma (4%) and CD (4%). This supports the view of LG as a morphological manifestation of more than one pathological process, involving a range of etiological factors, some of which may yet to be identified [2].
We have demonstrated the feasibility of performing TCR clonality studies on small, formalin-fixed and routinely processed biopsy specimens. However, poor DNA quality precluded PCR analysis in 28% of cases, highlighting the necessity for testing DNA quality priorto TCR analysis. Significantly, 15% (5/34) of LG cases from which quality DNA was obtained showed a monoclonal band on TCR-? gene re-arrangement analysis. Amongst all Hp positive patients with quality DNA, 43% (3/5) showed a monoclonal band.
Hp infection has been proposed as an inciting agent for LG, inducing the infiltration of T-lymphocytes into the gastric surface epithelium [9] and our findings suggest that in a significant proportionof these cases a clonal expansion of T-cells may develop. Of note, one Hp positive case displaying a monoclonal T-cell expansion showed clinical, endoscopic and histological improvement of LG following Hp treatment and this was associated with the development of a polyclonal T-cell population following repeat TCR-? analysis. This finding is in keeping with a study by Niemela which showed a decrease in number of IEL in LG following Hp treatment.19 However, 40% (2/5) of the monoclonal cases had no recognised disease association, suggesting other host-related factors may play a role. One of these cases had persistence of LG despite steroid treatment
Figure 2: Polyacrylamide gel of TCR-&gamma PCR products. Lane 1: molecular weight markers (100-400-bp); Lane 2: polyclonal control (without template DNA); Lane 3-4: Case X (monoclonal); Lane 5-6: Case Y (monoclonal); and Lanes 7 and 8: Case Z (polyclonal).
To date, none of the patients in this series have developed lymphoma although follow-up continues. The clinical significance of a clonal T-cell expansion in LG is not known and larger scale long-term follow-up studies are needed. In certain conditions, such as EATL, the presence of a monoclonal lymphoid population may be used to support a diagnosis of lymphoma when identified in association with appropriate histopathological features and/or clinical context. However CD8+ T cell lymphocytosis, EBV-driven immune reactions and cutaneous T cell proliferations can show a clonal expansion of lymphocytes and, lymphoid clones in other conditions, for example monoclonal gammopathy of uncertain significance and lymphoepithelial salivary gland lesions in Sjögren's syndrome, show no evidence of malignancy even after prolonged follow-up [20-22]. Thus clonality is not synonymous with malignancy/neoplasia [20,23].
In addition, although Hp is an established risk factor forgastric mucosa-associated lymphoid tissue (MALT) lymphoma and monoclonal B cell populations have been demonstrated in approximately 10-15% of Hp-associated gastritis, on follow-up these B-cell clones do not predictably evolve into malignant lymphoma. Saxena et al [23] found that B-cell clonal bands in Hp-associated gastritis which were associated with a background polyclonal smear and were irreproducible, were more likely to be reactive processes on follow-up. In contrast, 'pure' B-cell clonal bands have been shown to be specific for gastric lymphoma [24].
Less is known about T-cell clones in gastritis. Although detection of a monoclonal intra-epithelial T cell population in ulcerative jejunitis and refractory CD has been shown to be associated with higher risk of EATL, it is possible that clonal T-cells in LG may just be an indolent stage in its natural history and may not mean progression to lymphoma or identify patients at risk. It may be appropriate to consider cases of LG associated with a monoclonal T-cell band as a 'clonal disorder of uncertain malignant potential'. This term has been coined by some authors for clonal lesions which are not malignant and in which the development of lymphoma would require additional genetic events [20,23]. This multistep transformation over time is by no means inevitable, and it is possible that the presence of a clonal population is a transient and reversible event which may subside upon loss of antigenic stimulation, for example after Hp eradication.
In conclusion, immunohistochemical analysis and TCR gene rearrangement tests are potentially useful adjuncts in the diagnosticevaluations of LG, including in biopsy specimens. This case series shows a monoclonal expansion of T cells (CD3+, CD4-, CD8+) in a significant proportion of LG cases (5/34). Of note, 43% of the monoclonal cases were Hp positive compared with 21% of the polyclonal group. No other specific clinical or morphological characteristics were identified. On follow-up (median 64 months) no cases have developed lymphoma. Since the malignant potential of a monoclonal T cell population in LG remains uncertain, we would recommend repeat biopsy and close follow-up in patients with LG associated with a monoclonal T cell band and/or Hp infection as a precaution. In addition, all patients diagnosed with LG should betested and adequately treated for Hp infection.
Acknowledgement
We thank Dr Stuart Bloom and Dr Matthew Banks for providing clinical information on LG patients. This study was supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
References
- Haot J, Hamichi L, Wallez L, Mainguet P. Lymphocytic gastritis: a newly described entity: a retrospective endoscopic and histological study. Gut. 1988; 29: 1258-1264.
- Oberhuber G, Bodingbauer M, Mosberger I, Stolte M, Vogelsang H. High proportion of granzyme B-positive (activated) intraepithelial and lamina propria lymphocytes in lymphocytic gastritis. Am J Surg Pathol. 1998; 22: 450-458.
- Lynch DA, Sobala GM, Dixon MF, Gledhill A, Jackson P. Lymphocytic gastritis and associated small bowel disease: a diffuse lymphocytic gastroenteropathy? J Clin Pathol. 1995; 48: 939-945.
- Lynch DA, Dixon MF, Axon AT . Diagnostic criteria in lymphocytic gastritis. Gastroenterology. 1997; 112: 1426-1427
- Jaskiewicz K, Price SK, Zak J, Louwrens HD. Lymphocytic gastritis in nonulcer dyspepsia. Dig Dis Sci. 1991; 36: 1079-1083.
- Dixon MF, Wyatt JI, Burke DA, Rathbone BJ. Lymphocytic gastritis--relationship to Campylobacter pylori infection. J Pathol. 1988; 154: 125-132.
- Mäkinen JM, Niemelä S, Kerola T, Lehtola J, Karttunen TJ. Epithelial cell proliferation and glandular atrophy in lymphocytic gastritis: effect of H pylori treatment. World J Gastroenterol. 2003; 9: 2706-2710.
- Haot J, Jouret A, Willette M, Gossuin A, Mainguet P. Lymphocytic gastritis--prospective study of its relationship with varioliform gastritis. Gut. 1990; 31: 282-285.
- Niemelä S, Karttunen T, Kerola T, Karttunen R. Ten year follow up study of lymphocytic gastritis: further evidence on Helicobacter pylori as a cause of lymphocytic gastritis and corpus gastritis. J Clin Pathol. 1995; 48: 1111-1116.
- AP, Wyatt J, Jack AS, Dixon MF. Lymphocytic gastritis, gastric adenocarcinoma, and primary gastric lymphoma. J Clin Pathol. 1994; 47: 1123-1124.
- Miettinen A, Karttunen TJ, Alavaikko M. Lymphocytic gastritis and Helicobacter pylori infection in gastric lymphoma. Gut. 1995; 37: 471-476.
- Wolber R, Owen D, DelBuono L, Appelman H, Freeman H. Lymphocytic gastritis in patients with celiac sprue or spruelike intestinal disease. Gastroenterology. 1990; 98: 310-315.
- De Giacomo C, Gianatti A, Negrini R, Perotti P, Bawa P. Lymphocytic gastritis: a positive relationship with celiac disease. J Pediatr. 1994; 124: 57-62.
- Karttunen T, Niemelä S. Lymphocytic gastritis and coeliac disease. J Clin Pathol. 1990; 43: 436-437.
- Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000; 356: 203-208.
- Bagdi E, Diss T, Munson P, Isaacson PG. Mucosal Intra-epithelial Lymphocytes in Enteropathy-Associated T-cell Lymphoma, Ulcerative Jejunitis, and Refractory Celiac Disease Constitutes a Neoplastic Population. Blood. 1999; 94: 260-264.
- Verkarre V, Asnafi V, Lecomte T, Patey Mariaud-de Serre N, Leborgne M. Refractory coeliac sprue is a diffuse gastrointestinal disease. Gut. 2003; 52: 205-211.
- van Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003; 17: 2257-2317.
- S, Karttunen TJ, Kerola T. Treatment of Helicobacter pylori in patients with lymphocytic gastritis. Hepatogastroenterology. 2001; 48: 1176-1178.
- Collins RD. Is clonality equivalent to malignancy: specifically, is immunoglobulin gene rearrangement diagnostic of malignant lymphoma? Hum Pathol. 1997; 28: 757-759.
- Kyle RA. 'Benign' monoclonal gammopathy. A misnomer? JAMA. 1984; 251: 1849-1854.
- Quintana PG, Kapadia SB, Bahler DW, Johnson JT, Swerdlow SH. Salivary gland lymphoid infiltrates associated with lymphoepithelial lesions: a clinicopathologic, immunophenotypic, and genotypic study. Hum Pathol. 1997; 28: 850-861
- Saxena A, Moshynska O, Kanthan R, Bhutani M, Maksymiuk AW. Distinct B-cell clonal bands in Helicobacter pylori gastritis with lymphoid hyperplasia. J Pathol. 2000; 190: 47-54.
- Torlakovic E, Cherwitz DL, Jessurun J, Scholes J, McGlennen R. B-cell gene rearrangement in benign and malignant lymphoid proliferations of mucosa-associated lymphoid tissue and lymph nodes. Hum Pathol. 1997; 28: 166-173.