Case Report
Austin J Clin Pathol. 2014;1(2): 1007.
Eosinophilic Perifolliculitis Presenting as an Incidental Finding in a Patient with Leiomyomata with Perinodular Hydropic Degeneration
Essel Marie B de Leon*, Nicole Riddle and Philip Valente
Department of Pathology, University of Texas, USA
*Corresponding author: Essel Marie B de Leon, Department of Pathology, University of Texas, Health Science Center, Mail Code 7750, 7703 Floyd Curl Drive, San Antonio, Texas, 78229-3900, USA
Received: April 10, 2014; Accepted: May 02, 2014; Published: May 06, 2014
Abstract
Eosinophilic perifolliculitis is rare disease entity and is considered a possible variant of autoimmune oophoritis. Here we report a 49–year–old, nulliparous female presented with menorrhagia and markedly enlarged uterus. Incidentally, the right ovary showed numerous eosinophils infiltrating a hemorrhagic corpus luteum consistent with eosinophilic perifolliculitis. It is important to recognize eosinophilic perifolliculitis histologically because of its possible relation to autoimmune oophoritis which can cause primary ovarian failure.
Keywords: Eosinophilic perifolliculitis; Autoimmune oophoritis; Primary ovarian failure
Abbreviations
CD – Cluster of Differentiation; HPF – High Power Field; FSH – High Follicle–Stimulating Level; IL – Interleukin
Background
Eosinophilic perifolliculitis is a rare disease entity and is considered a possible variant of autoimmune oophoritis. With minimal discussion in the surgical pathology and gynecologic literature, the pathogenesis and natural history of eosinophilic perifolliculitis are not well–known. Some of the reported cases in the literature have been associated with autoimmune diseases.
Case Presentation
A 49–year–old, nulliparous female presented with menorrhagia and an enlarged uterus suspicious for leiomyosarcoma or endometrial tumor. Pelvic and transabdominal ultrasound revealed a markedly enlarged uterus equivalent to mid–term pregnancy measuring 21.0 x 11.0 x 17.6 cm. The right ovary is enlarged measuring 6.7 4.4 x 4 cm with no evidence of cystic change or mass. The left ovary is not visualized. Subsequently, she underwent total abdominal hysterectomy with bilateral salphingo–oophorectomy.
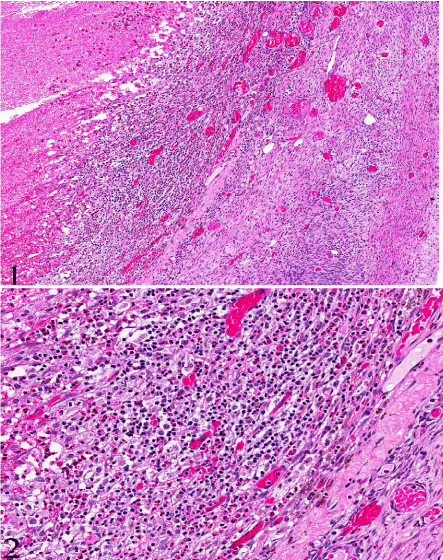
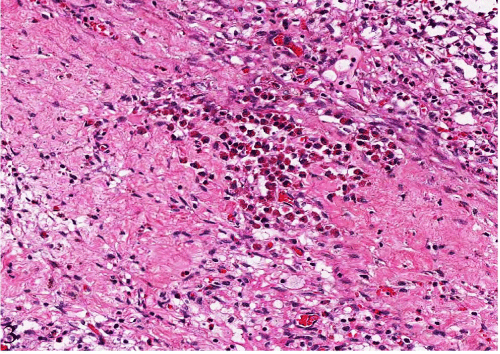
Gross examination showed an enlarged uterus weighing 2150 grams and a huge mostly intramural mass with multinodular whitetan fleshy areas with myxoid and cystic change measuring 17.0 x 9.0 x 4.5 cm. The cut–surface had multiple smaller nodules; some of the nodules were separated by mucoid gelatinous soft tissue. There were no areas of hemorrhage, calcification, or necrosis present. The endometrial cavity was distorted, compressed and lined by unremarkable endometrium with a small benign endometrial polyp. The bilateral ovaries and fallopian tubes appeared grossly unremarkable. Histologically, the uterus showed benign leiomyomata with perinodular hydropic degeneration and myxoid change. The perinodular hydropic change was characterized by multiple hypercellular nodules with blood vessels surrounded by hypocellular myxoid change. There was no cytologic atypia with rare mitotic activity (<1 mitosis⁄10HPF). The hydropic change within leiomyoma with had some thick–walled blood vessels and thin cords of residual smooth muscle cords in with an edematous pale background. The presence of extensive hydropic change may raise the suspicion for myxoid leiomyosarcoma in some difficult cases. This may also explain the clinical–radiologic suspicion of leiomyosarcoma. Incidentally, the right ovary showed abundant inflammatory cells predominantly eosinophils infiltrating a hemorrhagic corpus luteum only. There are no prominent primordial follicles present. The ovarian stroma was not involved by the inflammatory infiltrates.
Figure 1&2: Hemorrhagic corpus luteum surrounded by infiltrate of abundant eosinophils, lymphocytes, macrophages and plasma cells. (H&E, original magnification x 20 [1] and x 200 [2]).
Figure 3: Numerous eosinophils infiltrating the hemorrhagic corpus luteum (H&E, original magnification x 200 [3]).
Discussion
The histopathologic findings in the ovary are consistent with eosinophilic perifolliculitis which is considered to be a variant of autoimmune oophoritis. Autoimmune oophoritis is characterized by autoimmune inflammation of ovaries resulting in atrophy and fibrosis [1]. There is subsequent ovarian failure resulting in loss of fertility and ovarian hormone function. Since ovarian biopsies are not routinely performed in women with infertility work–up, there is little information on the pathology of autoimmune oophoritis. The histopathologic features described are characterized by lymphocytic and plasmacytic infiltrate with destruction of the developing or maturing follicle and sparing of primordial follicle often associated with cysts [1,2,3]. Acute oophoritis and tuberculous oophoritis maybe remotely considered in this case but these involve the entire ovarian parenchyma including the primordial follicle and have neutrophilic and lymphocytic infiltrates respectively.
The pathogenic mechanism of autoimmune oophoritis has not been well studied [3]. The immunophenotype of the ovarian inflammatory cells infiltrates revealed a mixture of B cells, plasma cells, T cells, macrophages and natural killer cells suggesting acomplex immune process with interaction of humoral and cellular mechanisms is involved in the pathogenesis [4]. There is some evidence that CD4 type 1 helper cells are the major pathogenic T–cell [3]. In addition, there is evidence that CD4 T helper 2 cells also elicit autoimmune oophoritis with autoreactive T cell producing IL–4 and IL–5 which are key mediators in eosinophil activation [3]. This mechanism elucidates the mechanism of eosinophilic perifolliculitis and its relation to autoimmune oophoritis. It also explains the few cases with predominant of eosinophilic infiltrates [5,6] including this case. Interestingly, enteric nematodes (rodent pinworm) which can potentially cause eosinophilia or eosinophilic infiltrate have been implicated in the pathogenesis of the autoimmune oophoritis in neonatal mice [3].
In general, patients with autoimmune oophoritis have a low estradiol level due to destruction of theca cells with preservation of granulosa cells which will subsequently results in increase in high follicle–stimulating level (FSH). The increase in FSH will then stimulate viable granulosa cells to produce inhibin thus, increasing serum inhibin concentration [7]. Steroid cell autoantibodies and⁄ or 17alpha–hydroxylase autoantibodies and/or cytochrome P450 side–chain cleavage autoantibodies and adrenal autoantibodies are consistently present in patients with autoimmune oophoritis [7]. Various ovarian specific type antibodies have been reported including microsomal, granulosa cell, theca cells, zona pellucida and oocytes antibodies [3].
Since eosinophilic perifolliculitis has been regarded as a variant of autoimmune oophoritis which can cause premature ovarian failure, most of the patients present with oligomenorrhea or secondary amenorrhea. The ovaries maybe normal or enlarged with cysts in the early stage and later become small and fibrocystic in the late stage. In our case, the patient presented with menorrhagia and an enlarged uterus highly suspicious for malignancy; and the eosinophilic perifolliculitis is an incidental finding. The menorrhagia may be explained by the presence of leiomyomas. Her ovaries were grossly unremarkable as may be seen in the early stage of autoimmune oophoritis.
Autoimmune oophoritis typically occurs in the setting of autoimmune polyendocrine syndromes and is commonly associated with Addison’s disease, hypothyroidism, hyperparathyroidism and diabetes mellitus [1]. Although our patient clinical history is not significant for other autoimmune diseases and the presence of circulating autoantibodies is unconfirmed, this case may represent a variant autoimmune oophoritis. Her father having celiac disease is of interest given that autoimmune diseases have a strong hereditary component and may cluster in families as different illnesses. It is essential to recognize eosinophilic perifolliculitis histologically because of its possible relation to autoimmune oophoritis which can cause primary ovarian failure and its frequent association other autoimmune diseases.
References
- Varras M, Anastasiadis A, Panelos J, Balassi E, Demou A, Akrivis CH. Autoimmune oophoritis: Clinical presentation of an unusual clinical entity. OA Case Reports. 2013; 31; 2: 7.
- Suh YL. Autoimmune oophoritis--a case report. J Korean Med Sci. 1992; 7: 284-290.
- Mackay I, Rose N: The Autoimmune Disease, 4th edn: Oophoritis, Elsevier. 2006; 849-858
- Sedmak DD, Hart WR, Tubbs RR. Autoimmune oophoritis: a histopathologic study of involved ovaries with immunologic characterization of the mononuclear cell infiltrate. Int J Gynecol Pathol. 1987; 6: 73-81.
- Page K, Pagidas K, Derosa MC, Quddus MR. Eosinophilic perifolliculitis presenting as a painful cystic ovarian mass in a woman with fibromyalgia: a case report. J Reprod Med. 2006; 51: 141-144.
- Lewis J. Eosinophilic perifolliculitis: a variant of autoimmune oophoritis? Int J Gynecol Pathol. 1993; 12: 360-364.
- Tsigkou A, Marzotti S, Borges L, Brozzetti A, Reis F, Candeloro P, et al. High serum inhibin concentration discriminates autoimmune oophoritis from other forms of primary ovarian insufficiency. J Clin Endocrinol Metab. 2008; 93: 1263-1269.