Case Report
Austin J Clin Pathol. 2014;1(3): 1015.
Clear Cell Renal Cell Carcinoma with Melanocytic Differentiation: A Case Report, with Review of the Literature
Kiran Krishne Gowda, Parimal Agrawal and Kim Vaiphei*
Department of Histopathology, Post Graduate Institute of Medical Education and Research, India
*Corresponding author: Kim Vaiphei, Department of Histopathology, Post Graduate Institute of Medical Education and Research, (PGIMER), Chandigarh, India
Received: July 08, 2014; Accepted: Aug 04, 2014; Published: Aug 05, 2014
Abstract
Renal cell carcinoma consists of several subtypes, each of which has its own clinical features, and cytogenetic and molecular characteristics. Recognizing histologic patterns of RCC is important not only for correct diagnosis, but also for providing insight into biological behavior of the tumor and subsequent appropriate medical care for the patient. Pigments other than hemosiderin have been observed in renal cell carcinoma. It has been described in both clear cell and chromophobe subtypes of renal cell carcinoma. The nature of these pigmentations is consistent with melanin, neuromelanin, and lipochrome granules. We report a rare case of pigmented clear cell renal carcinoma with melanocytic differentiation. A review of cases of pigmented clear cell renal cell carcinoma in literature is also presented. Awareness of pigmentation in primary renal cell carcinoma is important in differentiating it from primary and metastatic malignant melanoma and other malignancies that can show pigmentation.
Keywords: Renal cell carcinoma; Pigment; Melanin
Abbreviations
RCC: Renal Cell Carcinoma; EMA: Epithelial Membrane Antigen; PEComa: Perivascular Epithelioid Cell Tumor
Introduction
Renal cell carcinoma (RCC) accounts for approximately 3% of adult malignancies and 0.1% to 0.3% of all pediatric neoplasms with 90%-95% of neoplasms arising from the kidney [1]. RCC has been classified into ten histologic subtypes: clear cell, multilocular clear cell, papillary, chromophobe, carcinoma of the collecting ducts of Bellini, renal medullary carcinoma, Xp11 translocation carcinomas, carcinoma associated with neuroblastoma, mucinous tubular and spindle cell carcinoma and renal cell carcinoma unclassified. Mixture of these distinct types is not infrequent. In addition, pigment other than hemosiderin has been documented in different types of RCC in adults [2-8] expanding the spectrum of the histologic features of RCC and increasing the difficulty of diagnosing the tumor. We report the first case of clear cell RCC exhibiting melanocytic differentiation in pediatric age group.
Case Presentation
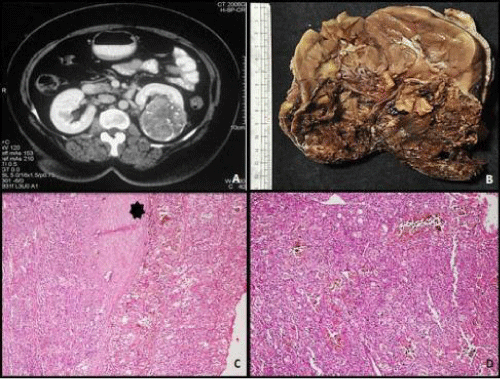
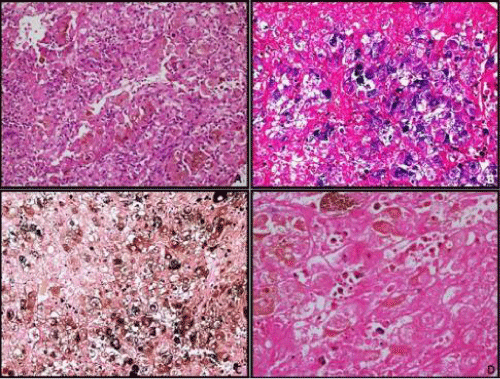
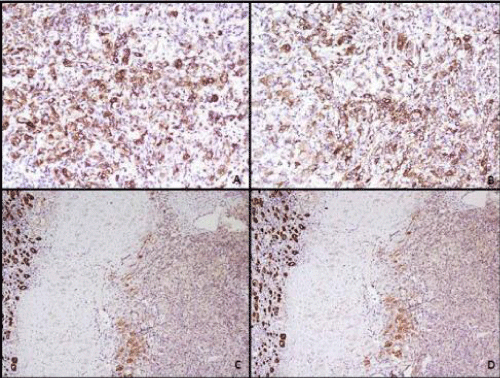
A 12-year-old female child presented with complaints of abdominal pain. Abdomen examination revealed a soft tender mass measuring 4 cm in left lumbar region. Ultrasonography of abdomen revealed a mass in lower pole of left kidney which was confirmed on CT scan (Figure 1A). After presurgical evaluation, patient underwent radical nephrectomy which consisted of left kidney measuring 14 x 10 x 8 cm and ureter measuring 6.5 cm in length. Cut surface showed a circumscribed dark brown to tan tumor in lower pole measuring 6x5.5x5 cm, without any capsular breach (Figure 1B). Tumor was solid with focal areas of cystic degeneration (10% of total area). The remaining renal parenchyma appeared normal including pelvicalyceal system. On microscopy tumor cells were arranged in solid, alveolar and acinar growth pattern with rich sinusoidal network of delicate vascular channels (Figure 1D). Tumor was composed of cells with abundant clear-to-granular cytoplasm and centrally located nuclei with single prominent nucleoli (Fuhrman nuclear grade-III/ IV). In majority of tumor cells, dark brown pigment was observed in cytoplasm. The pigment was dark brown, granular, nonrefractile and stained blue-green with Schmorl's and black with Masson Fontana stain indicating it to be melanin (Figure 2). Immunostains for HMB- 45 and S-100 stained most of tumor cells positively, while cytokeratin and epithelial membrane antigen (EMA) were negative confirming melanocytic differentiation (Figure 3). Renal capsule was free of tumor tissue. The renal vessels and ureter were free of disease. A diagnosis of clear cell RCC with melanocytic differentiation (Furhman nuclear grade- III/IV; TNM stage I) of kidney was made. The patient is alive and well at 7 months follow-up.
Figure 1: A: Abdominal CT scan showing a mass in the lower pole of the left kidney; B: Gross picture of the mass showing well-defined solid tumor with blackish areas having well defined margin; C: Histology of the tumor showing a well circumscribed tumor (H&E, x200); D: Microphotograph of the tumor to show the tumor cells arranged in diffuse sheet (H&E, x200).
Figure 2: A: Tumor cells showed moderate amount of clear to granular eosinophillic cytoplasm having mildly pleomorphic nuclei with occasional prominent nucleoli. There was abundant amount of intracytoplasmic brown pigment in almost every tumor cell (H&E, x200). B: microphotograph of the tumor cells showing blue color staining of the pigment on Schmorl stain (x200); C: microphotograph to show black color staining of the pigment on Masson Fontana stain (x200); D: the brown pigment was negative with Prussian blue stain (x400).
Figure 3: Photomicrographs of the immunohistochemistry staining showing - A: most of the tumor cells positive for HMB-45 (peroxidase anti-peroxidase, x200) and B: for S-100 (peroxidase anti-peroxidase, x200); C: negative for pancytokeratin (peroxidase anti-peroxidase, x100) and D: epithelial membrane antigen (peroxidase anti-peroxidase, x100). Renal tubular epithelial cells are showing positive staining for pancytokeratin and epithelial membrane antigen.
Discussion
RCC is a rare disease in children and adolescents. Speculation exists about whether RCC in children represents a different entity from its adult counterpart. However, the overall prognosis of children seems similar to that of adult patients [9]. As acknowledged by the 2004 WHO classification of adult kidney tumors, different subtypes of RCC have different clinical outcomes and show different response to therapy. RCC originate from the renal proximal tubules, with many morphologic types identified based on morphology, histochemistry, clinical behavior, and genetic alterations. Among the subtypes clear cell renal carcinoma, papillary renal carcinoma and chromophobe renal carcinoma are most frequently seen and constitute 95% of adult RCC. Clear cell carcinoma alone accounts for 75% of adult RCC [1] and 58.5% of pediatric RCC [9].
The finding of a pigmented renal cell tumor is an uncommon occurrence, which represents a diagnostic challenge requiring a differential diagnosis with other primary and secondary pigmented neoplasms of the kidney. Dark brown endogenous pigments found in renal cell carcinoma include hemosiderin, melanin, homogentisic acid and lipofuchsin. Hemosiderin granules are variably sized, reflective and golden brown and can be confirmed with Perl's stain. Lipofuschin shows perinuclear location. Homogentisic acid is an endogenous, brown-black pigment, which presents in the skin, connective tissue, and cartilage of patients with alkaptonuria, a rare metabolic disease. Our patient did not have alkaptonuria, and the pigment existed only in the tumor cells. Special stains further ruled out the other possibilities mentioned.
Non-hemosiderotic pigmentation in renal cell carcinoma has been described primarily in two variants, the clear cell and the chromophobe RCC. The first report of pigmentation in clear cell carcinoma came from Kamishima et al. [2], in which they attributed the pigments to abnormal lysosomes, with histochemical features consistent with neuromelanin. This was followed by more cases from the same group of authors. Fukuda et al. [3] reported neuromelanin in 3 out of 5 cases; a finding that suggested the lysosomal origin of granules in those three cases. One of these three cases was chromophobe RCC. A total of 7 cases (including current case) of pigmented clear cell RCC with intracytoplasmic pigment deposition have been reported in English literature [2-8]. Melanocytic differentiation as seen in our case in the form of HMB-45 and S-100 expression by tumor cells has been documented in only one other case [5]. Michal et al. described cases of a particular renal carcinoma entity originally named pigmented microcystic chromophobe cell carcinoma. However, the pigment deposits were mostly extracellular [8]. Pigment in our case is composed of dark brown, non-refractile, fine granules and stained black with Masson Fontana, consistent with melanin. The tumor cells stained positively with HMB45 and S-100 but not for cytokeratins and EMA indicating melanocytic differentiation of tumor cells. This is the first case of clear cell RCC with melanocytic differentiation in pediatric age group.
Of all the cases of pigmented clear cell RCC reported so far, only one showed distant metastasis [6]. It is interesting to note that the ages of presentation of pigmented RCC are younger (Table 1) compared with the usual age of conventional non-pigmented RCC, which is sixth decade of life [1]. Our case is the youngest of all the pigmented RCC, presenting at 12 years of age. Apparently, more studies are needed to support this observation. It is important to recognize the presence of these pigments in renal cell carcinoma and differentiate it from metastatic deposits in the kidney. Metastatic melanoma, neuroendocrine neoplasm, neuroectodermal tumors, pigmented perivascular epithelioid clear cell tumor (PEComa), and pigmented pheochromocytoma are the most important considerations in the differential diagnosis. The negative staining of tumor cells for synaptophysin and Chromogranin A ruled out the diagnosis of pheochromocytoma as well as neuroectodermal and neuroendocrine tumors. The significance of pigment in renal cell carcinoma other than differential diagnosis is yet to be established.
Authors
Age/ Sex
Diagnosis
Type of
pigment
Special stains
IHC
Electron microscopy
Follow-up
Kamishima et al. 1995
38/ F
CRCC
Neuromelanin
PAS/PASD+ MF+, PB-
Schmorl+
HMB45- S100-
No melanosome
A/W-
8 mon
Fukuda
et al. 1997
61/ M
CRCC
Neuromelanin
PAS/PASD+ MF+, PB-
HMB45- S100-
No melanosomes
A/W-
52mon
Hirokawa et al. 1998
40/ F
CRCC
Neuromelanin
PAS/PASD+ MF+, PB-
Schmorl+
HMB45- S100-
N/A
N/A
Lei et al. 2001
26/ F
CRCC
Melanin
MF+
HMB45+ S-100+, CK-
N/A
A/M-
15mon
Rossi
et al. 2009
48/ M
CRCC
Neuromelanin
PAS/PASD+ MF+, PB-
HMB 45- S100-
No melanosomes
A/W-
4mon
Matalka et al. 2013
27/ F
CRCC
Neuromelanin
PAS/PASD+ MF+, PB-
HMB45- S100-
No melanosomes
A/W-
120mon
Current case
12/F
CRCC
Melanin
PAS/PASD+ MF+, PB-
Schmorl+
HMB45+ S-100+ CK-, EMA-
N/A
A/W-
7mon
Table 1: Reported cases of pigmented clear cell renal cell carcinoma and review of literature.
In summary, we report a rare case of pigmented clear cell RCC with melanocytic differentiation, a first in pediatric age group. This feature expands the spectrum of morphological changes of RCC. Whether pigmented clear cell RCC behave differently from more common non- pigmented variant is difficult to ascertain due to rarity of the condition. Further molecular and cytogenetic studies are needed on these types of pigmented clear cell RCC.
References
- Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and genetics. Tumors of the urinary system and male genital organs. Lyon: IARC Press. 2004.
- Kamishima T, Fukuda T, Emura I, Tanigawa T, Naito M. Pigmented renal cell carcinoma. See comment in PubMed Commons below Am J Surg Pathol. 1995; 19: 350-356.
- Fukuda T, Kamishima T, Emura I, Takastuka H, Suzuki T. Pigmented renal cell carcinoma: accumulation of abnormal lysosomal granules. See comment in PubMed Commons below Histopathology. 1997; 31: 38-46.
- Hirokawa M, Sakurai T, Shimizu M, Manabe T, Kanahara T. Cytology of pigmented renal cell carcinoma. A case report. See comment in PubMed Commons below Acta Cytol. 1998; 42: 788-790.
- Lei JY, Middleton LP, Guo XD, Duray PH, McWilliams G, Linehan WM, et al. Pigmented renal clear cell carcinoma with melanocytic differentiation. See comment in PubMed Commons below Hum Pathol. 2001; 32: 233-236.
- Rossi G, Cadioli A, Costantini M, Grazia Del Buono M, Oleari G. Heavily pigmented renal cell carcinoma: a case report, with review of the literature and differential diagnosis. See comment in PubMed Commons below Int J Surg Pathol. 2009; 17: 167-169.
- Matalka I, Al-Hussaini M, Hani I B. Pigmented Renal Cell Carcinoma: A case with unusual findings and review of the literature. J Med J. 2013; 47: 176-182.
- Michal M, Hes O, Svec A, Ludvíková M. Pigmented microcystic chromophobe cell carcinoma: a unique variant of renal cell carcinoma. See comment in PubMed Commons below Ann Diagn Pathol. 1998; 2: 149-153.
- Indolfi P, Terenziani M, Casale F, Carli M, Bisogno G, Schiavetti A, et al. Renal cell carcinoma in children: a clinicopathologic study. See comment in PubMed Commons below J Clin Oncol. 2003; 21: 530-535.