1Department of Pathology, University of Louisville, USA
2Department of R&D, Timac Agro International-Roullier Group, Spain
3Department of Chemistry and Soil Chemistry, University of Navarra, Spain
4Research Resource Center, University of Louisville, USA
5Department of R&D, Timac Agro International, France
*Corresponding author: Vetvicka V, Department of Pathology, University of Louisville, 511 S. Floyd, Louisville, KY 40202 USA
Received: September 17, 2014; Accepted: November 10, 2014; Published: November 11, 2014
Citation: Vetvicka V, Garcia-Mina JM, Proctor M and Yvin J-C. Synergistic Effects of Humic Acid and Glucan in Hepatoprotection against Experimental Liver Injury. Austin J Clin Pathol. 2014;1(4): 1020. ISSN : 2381-9170
Despite common presence of humic acid, our full knowledge of its biological effect is still lacking. In this paper, we studied the effects of either humic acid alone or in combination with glucan, on reduction of liver damage caused by two different agents, ethanol or lipopolysaccharide. In all tested parameters, two samples of humic acid managed to ameliorate the damaging effects of either lipopolysaccharide or ethanol treatment. In addition, a humic acid-glucan combination showed stronger effects that humic acid alone.
Keywords: Humic acids; Glucan; Liver; Hepatotoxicity; LPS; Ethanol
Liver regulates numerous important functions in the organism, serving as the main organ for detoxification of various substances. The pathogenesis of liver damage involves many cell types in liver via cell death and regeneration processes. The liver damage often progresses from acute to chronic hepatitis, fibrosis, cirrhosis and carcinoma. Several experimental models of liver damage are firmly established, including carbon tetrachloride [1], LPS damage [2] and ethanol [3]. Particularly important are studies of alcoholic liver disorders, as they are the leading causes of morbidity throughout the world. Individual step include fatty liver and subsequent alcoholic hepatitis [4], which leads to cirrhosis [5]. All this makes the search for highly effective drugs with hepatoprotective effects an important problem for both medicine and pharmacology.
Humic acids are ubiquitous molecules which can be found wherever organic matter is being decomposed or transposed. Despite long knowledge of humic acids, some of their health related effects remains controversial. Some studies showed antiviral properties [6], some stimulation of lymphocytes [7]. The effects of the abundance of oxygen alkyl-related groups on the biological effects of humic acid were found [8]. On the other hand, a chromosomal abnormalities resulting from higher doses of humic acid were described [9].
Glucans are members of a group of physiologically active compounds generally called biological response modifiers and represent highly conserved structural components of cell walls in yeast, fungi, seaweed and plants. Glucan’s role as a biologically active immunomodulator has been well documented for over 60 years. Biological effects of glucans include stimulation of infectious immunity, activation of bone marrow cell production, anti-cancer effects, lowering of blood cholesterol and amelioration of stress [10- 12, for review see 13].
Lately, several studies demonstrated that various natural bioactive molecules have better effects when mixed with carefully chosen additives. Studies showing synergistic effects of resveratrol and glucan [14] or glucan and vitamin C [15] suggested glucan as an optimal additive. In our preliminary studies, we found significant synergistic effects of humic acid and glucan [16]. In addition, Celik et al. found that adding humic acid and Saccharomyces cerevisiae extracts into the food positively influenced performance and some biochemical parameters of chicken [17]. In addition, these substances increase effects of vaccination against influenza [18]. As some of these studies suggested possible inhibition of liver damage, we studied the hepatoprotective effects of β-glucan, humic acid and their combination reported in this paper.
Female, 6 to 10 week old BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All animal work was done according to the University of Louisville IACUC protocol. Animals were sacrificed by CO2 asphyxiation.
Ethanol, lipopolysaccharide (from Escherichia coli), formalin, Limulus lysate test E-TOXATE, and polymixin B were purchased from Sigma (St. Louis, MO, USA).
We used a combination of manno-oligosaccharides and β-glucan extracted from Saccharomyces cerevisiae by autolysis at high temperature at controlled pH. When completed, the cell walls and extracts are separated by centrifugation and cell wall is spray dried. The glycosidic composition is 21% mannan, 24% β-glucan (Lallermand Animal Nutrition, Montreal, Canada).
Two lignin-derived organic systems were obtained from diverse organic materials using the methodology described by the International Humic substances Society (IHSS) to extract humic substances (HS) and humic acids (HA), as described in [19]. A first HA was extracted from black peat (Galicia, Spain) (HA8) and the other one from red Quebracho (Schinopsis sp.) barks (HA10). The functional organic carbon (C) distribution obtained from 13C-NMR analysis were: 27 % (aliphatic C); 24 % (O-alkyl C); 23 % (aromatic C); 9 % (phenol C); 13 % (carboxylic C) and 3 % (carbonylic C) for HA8, and 11 % (aliphatic C); 35 % (O-alkyl C); 29 % (aromatic C); 22 % (phenol C); 2.2 % (carboxylic C) and 0.5 % (carbonylic C) for HA10. Average molecular weights obtained from size-exclusion liquid chromatography analysis were 13929 Dalton for HA8 and 10963 Dalton for HA10 [19].
Hepatotoxicity was induced by oral feeding of ethanol (1 g/kg of body weight) for 10 days as described by [20] or by an ip. injection of 100 ng/kg body weight of lipopolysaccharide (LPS) as described by [2]. Alcohol was diluted in water, LPS in PBS. Mice were randomly divided into several groups and administered orally by gavage during 10 days as follows: Group 1 – control group treated with PBS; Group 2 – treated with glucan; Group 3 – treated with AH8; Group 4 – treated with AH10; and Group 5 – treated with a combination of glucan, AH8 and AH10. At the end of the study, blood was collected and serum prepared. After that, mice were sacrificed and livers were immediately excised and use either for homogenates or for histology.
The enzymatic activities of AST, ALT and ALP were assayed spectrophotometrically by (Antech Diagnostics, Louisville, KY, USA). Liver homogenate were prepared by the following technique: livers were excised and rinsed in saline. A small section from each liver was placed in 10% PBS-formalin solution to be used in histological slides. Rest was frozen in liquid nitrogen. Frozen liver was grounded to a fine powder and 20-25 mg of powder was solubilized. The GSH levels were measured by the GSH test kit (Dojindo Labs, Kumamoto, Japan), SOD as described by Prasanna and Purnima [21] and malondialdehyde (MDA) as shown in [22].
Mice were euthanized by inhalation of CO2 and euthanasia was confirmed by pneumothorax. Liver was removed and fixed with 10% buffered formalin. Fixed tissues were trimmed and paraffin-embedded for processing. The blocks were cut into 4 μm sections and stained with hematoxylin and eosin for histopathology examination. Same lobes of the livers were used for histopathological analysis.
Data were expressed as means ± SD. Statistical analysis was performed by a pair t-test using a GraphPad Prism 502 software (GraphPad Software, USA).Values of P ≤ 0.05 were considered statistically significant.
Both LPS and ethanol represent widely used models of experimental liver damage. All doses were based on previously published studies. Animals were randomly selected into six groups. In five groups, hepatotoxicity was induces by LPS injection. Four groups were treated with either glucan, two types of humic acid, or a combination of both humic acids and glucan, for a period of 10 days. After the end of the treatment, mice were sacrificed and blood and liver harvested. The results of LPS-induced hepatotoxicity are shown in Tables 1 and 3. Administration of LPS caused a marked increase in serum levels of AST, ALT and ALP. When we tested effects on hepatic enzymes, LPS-treatment caused increase of MDA, but strong decrease of levels of GSH and SOD. Treatment with glucan alone significantly improved levels of all tested parameters with exception of GSH. Both humic acid samples improved levels of all six tested markers, and their effects were comparable to those of glucan with the only exception being MDA, where glucan returned the elevated levels significantly more. In all cases, the effects of the glucan-humic acid combination were the strongest.
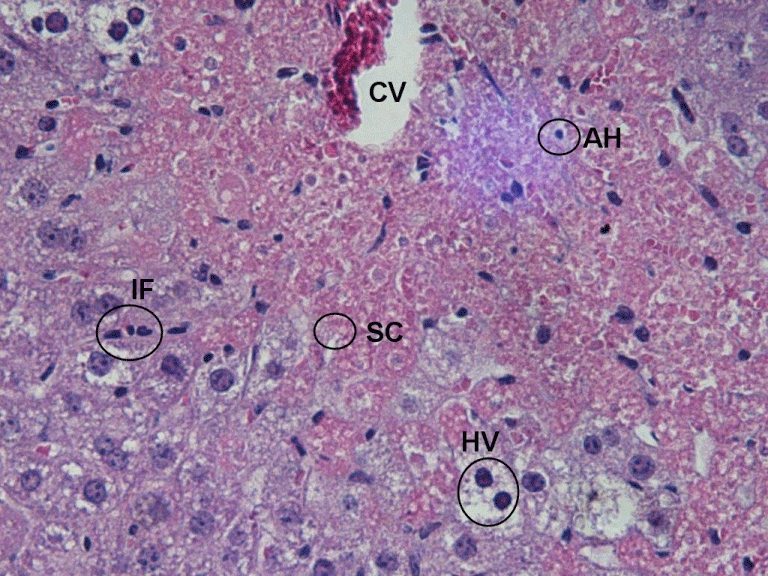
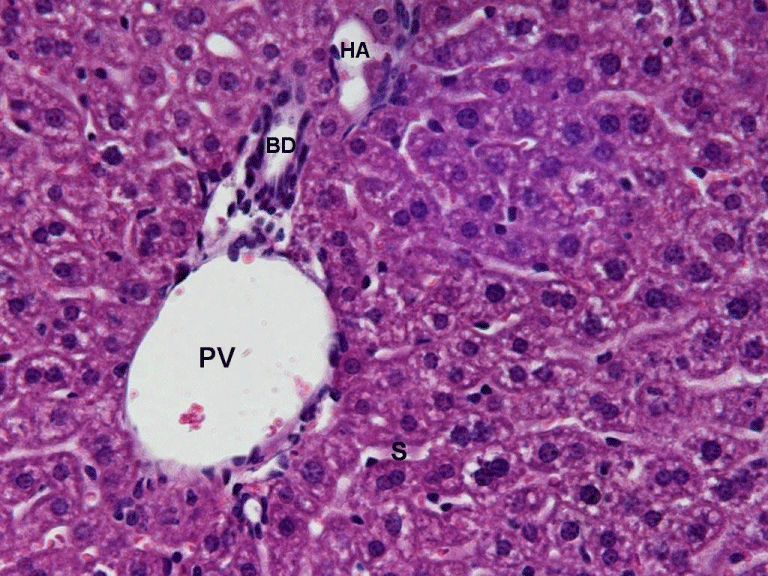
The same experimental design was used in case of ethanol-induced hepatotoxicity. Glucan treatment significantly lowered the effects of ethanol in case of ALT, ALP, MDA and SOD. Individual humic acids were similarly effective, the only nonsignificant effects being effects on AST in case of AH8. Similarly to LPS administration, the combination of glucan with humic acids produced the strongest effects, sometime up to three times stronger than individual components (Table 2 and 4). Our findings were confirmed by histological observations. Figure 1 reflecting the effects of the glucan-humic acid combination shows normal hepatic parenchyma, whereas Figure 2 (ethanol only) shows clear evidence of marked hepatocellular degeneration.
Both ethanol and LPS are well established models of hematotoxic damage of the liver. The close relation between alcohol and liver damage is mostly due to the fact that around 80% of ethanol is metabolized in the liver to the cytotoxic acetaldehyde, which is further oxidized by aldehyde oxidase resulting in various reactive oxygen species [23]. Additional damages are based on depletion of endogenous antioxidants and resulting oxidative stress [24].
Various herbal formulae or natural molecules are currently evaluated or even prescribed for various types of health problems, including liver damage [25,26]. Our preliminary study [8] suggested possible effects of humic acid on hepatotoxicity. In addition, we later confirmed this study on a CCL4-mediated liver damage (unpublished results). The aim of the current study was to evaluate the hypothesis that humic acid and glucan possess general hepatoprotective activity regardless the type of hepatic injury.
The mechanisms of the liver protection are currently unknown. As the increase of free radicals during alcohol-induced hepatotoxicity is expected to play an important role [27,28], it is possible that known antioxidant effects of glucan [29] and humic acid [30] are involved. Therefore, this makes us interested to evaluate the antioxidant effects of our samples. These activities were proven by significantly decreased level of total liver proteins.
It is established that increased levels of AST and ALT in the serum indicate damaged and/or necrosis of hepatocytes. In our study we showed that both LPS and ethanol significantly increased these levels, whereas humic acid and especially humic acid-glucan combination significantly decreased the level of these enzymes in the serum, suggesting a strong decrease of liver damage. The liver protecting effects might be caused by an augmentation of antioxidant enzyme protective system as they increase GSH levels compromised due to the liver toxicity. Similar mechanisms were previously suggested for restoration of GSH and CAT levels [3]. As exogenous GSH has no effects on hepatic GSH loss in damaged liver [31], the protective effects found in our study can be explained by GSH replenishment.
Therefore, our data showed that both humic acid and humic acid-glucan combination have robust hepatoprotective effects. This suggests that either humic acid alone or in combination with glucan is capable of preventing alcoholic liver damage by ameliorating alcohol-induced oxidative stress. The study evaluating the possible inhibition of inflammatory cytokines known to be involved in liver damage [32] is currently under progress.
Effects of glucan and humic acid on serum ALT, AST, and ALP of LPSinduced hepatotoxicity in mice.
Sample |
AST |
ALT |
ALP |
PBS |
73.2 ± 5.5 |
19.4 ± 3.1 |
21.2 ± 1.9 |
LPS |
177.7 ± 11.2 |
166.5 ± 17.2 |
188.2 ± 17.5 |
Glucan |
102.2*± 8.9 |
69.9*± 4.7 |
82.3*± 7.1 |
AH8 |
111.2*± 6.9 |
70.2*± 7.1 |
83.4*± 6.6 |
AH10 |
108.5*± 6.8 |
71.7*± 3.8 |
80.1*± 7.2 |
Glucan + AH8+AH10 |
81.2*± 4.7 |
33.1*± 2.9 |
28.9*± 2.5 |
*Significant difference against LPS group at P ≤ 0.05 level.
Effects of glucan and humic acid on serum ALT, AST, and ALP of ethanol-induced hepatotoxicity in mice.
Sample |
AST |
ALT |
ALP |
PBS |
80.8 ± 6.1 |
22.9 ± 1.2 |
25.5 ± 2.8 |
Ethanol |
341.9 ± 22.1 |
118.5 ± 3.9 |
268.8 ± 26.3 |
Glucan |
292.2 ± 11.7 |
54.4*± 3.7 |
101.2*± 8.8 |
AH8 |
301.1 ± 23.6 |
66.4*± 2.9 |
123.3*± 7.9 |
AH10 |
277.5*± 27.8 |
60.6*± 4.9 |
120.9*± 9.2 |
Glucan + AH8+AH10 |
172.2*± 15.5 |
33.4*± 2.1 |
46.4*± 2.7 |
*Significant difference against ethanol group at P≤ 0.05 level.
Effects of glucan and humic acid on level of hepatic enzymes GSH, MDA and SOD in LPS-induced hepatotoxicity.
Sample |
GSH |
MDA |
SOD |
PBS |
20.1 ± 1.9 |
20.1 ± 1.8 |
60.2 ± 5.5 |
LPS |
9.9 ± 0.8 |
77.8 ± 5.6 |
35.7 ± 3.1 |
Glucan |
13.4 ± 1.6 |
33.2*± 2.3 |
44.5*± 3.9 |
AH8 |
15.1*± 2.0 |
40.3*± 3.7 |
45.9*± 3.9 |
AH10 |
13.9*± 1.2 |
44.3* ± 1.7 |
41.1*± 4.0 |
Glucan + AH8+AH10 |
17.1*± 1.6 |
25.4*± 1.9 |
55.6*± 3.9 |
*Significant difference against LPS group at P ≤ 0.05 level.
Effects of glucan and humic acid on level of hepatic enzymes GSH, MDA and SOD in ethanol-induced hepatotoxicity.
Sample |
GSH |
MDA |
SOD |
PBS |
19.9 ± 1.9 |
8.8 ± 0.5 |
60.2 ± 5.8 |
Ethanol |
8.7 ± 2.2 |
71.1 ± 5.9 |
20.2 ± 3.1 |
Glucan |
14.2 ± 2.5 |
33.8*± 4.2 |
33.9*± 2.7 |
AH8 |
11.9*± 1.2 |
41.3*± 3.8 |
34.8*± 3.0 |
AH10 |
13.5*± 2.1 |
37.7*± 3.0 |
29.2*± 2.7 |
Glucan + AH8+AH10 |
16.6*± 2.0 |
20.8*± 1.8 |
44.9*± 3.5 |
*Significant difference against ethanol group at P≤ 0.05 level.
Normal hepatic parenchyma, consisting of cells that are large and polyhedral, with round nuclei and abundant heterochromatin and nucleoli. A typical portal triad displays a portal vein (PV), bile duct (BD), and hepatic artery (HA). Capillary sinusoids (S) are of normal caliber and there is no evidence of congestion. 400x.
Evidence of marked hepatocellular degeneration and distortion around the central vein (CV), Inflammatory cell infiltration (IF), and cytoplasmic vacuolization (HV) of hepatic cells. Sinusoidal congestion (SC) was also apparent. Apoptotic hepatocytes (AH) were also observed in areas of centrilobular necrosis. 400x.