
Review Article
Austin J Clin Pathol. 2015; 2(2): 1028.
Towards a Role for Clinical Pathology Diagnostics for Childhood Maltreatment
Bearer EL¹*, Juye J², Trickett P³, Kaplan CD³ and Mennen F³
1Department of Pathology, University of New Mexico Health Sciences Center, Albuquerque, NM, USA
2Department of Social Work, California State University, Fullerton, CA, USA
3School of Social Work, University of Southern California, Los Angeles, CA, USA
*Corresponding author: Elaine L. Bearer, University of New Mexico Health Sciences, Albuquerque, Albuquerque, New Mexico, NM, USA
Received: August 27, 2015; Accepted: September 09, 2015; Published: September 29, 2015
Abstract
Recent reports from the Center for Disease Control and Kaiser Permanente demonstrate that early life adverse experience leads to morbidity and mortality in adulthood. To date there are no objective tests that help care-givers or local child protective services make informed decisions for children with a history of abuse, neglect or trauma. This is the first report from a new group of transdisciplinary investigators describing a new approach to identify the biological impact of childhood maltreatment using clinical pathology testing. Such new quantitative measurements will be useful to identify children at risk for poor mental and physical health outcomes and to follow response to interventions.
Keywords: DNA methylation; MR imaging; Cortisol; Child abuse; Child maltreatment; Childhood neglect
Introduction
Early life events profoundly impact social, emotional, behavioral and biological well-being of the individual throughout the lifespan [1,2,3]. The Adverse Childhood Experience (ACE) study, performed by collaboration between Kaiser Permanente and the US government’s Center for Disease Control and Prevention questioned more than 17, 000 adults undergoing normal physical exams about childhood experiences, including abuse, neglect and family dysfunction. The ACE study results predict that certain childhood experiences are major risk factors for illness, substance use and mental health disorders, social problems and early death (Figure 1). Child abuse and neglect are among the most profound causes of ACE.
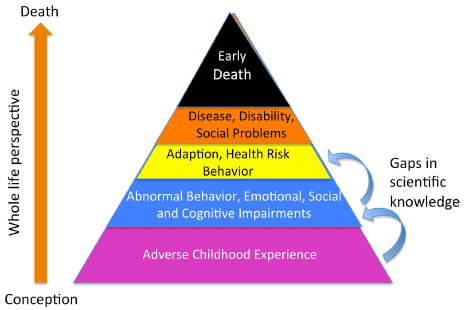
Figure 1: Showing the impact of adverse childhood experience on social wellbeing,
brain and behavioral health, and early death (adapted from the CDC).
Note the gap in understanding between adverse childhood experience and
adult outcomes. Clinical pathology objective testing can close this gap.
Childhood maltreatment in the US has reached crisis levels. Consider this: In 2013-2014 fiscal years, there were nearly 150,000 cases of child abuse and neglect investigated by the Los Angeles County Department of Children and Family Services [County of Los Angeles County. Department of Children and Family Services, 2013-2014 Biennial Report]. New Mexico ranked 26th in the nation in 2013 for deaths of children from abuse (18 deaths per 100,000) [Centers for Disease Control, National Center for Health Statistics]. While maltreatment may occur in any socioeconomic group, the more severe and chronic cases occur in low socioeconomic groups who are often members of ethnic minorities. Neither urban nor rural communities are immune from childhood neglect, abuse, or trauma.
A leading cause of mental illness is early life trauma. One in four children in the U.S. are affected with a serious neuropsychiatric illness. These illnesses is more severe in children, recur more frequently, tend to be chronic and persist into adulthood. More than half of adult mental illness begins in childhood. Mental illnesses cause and compound chronic medical illnesses. Treatments are generally inadequate. The annual economic impact in the US is in the hundreds of billions of dollars.
Early life adversity may come in a variety of forms: childhood neglect, physical, emotional and/or sexual abuse, family dysfunction, domestic violence, or even living in poverty and/or in a socially disadvantaged (“rough”) violent neighborhood [4]. There is some suggestion that the type, duration, and intensity of adversity affect the child’s development, biology, and mental/cognitive skills differently. Poverty alone can be a factor in early childhood development, partly due to the stress it provokes in the family [2]. Moreover, gender differences in child abuse patterns can result in long-term effects on the variation in profiles of populations with co-occurring mental health and substance use disorders presenting for treatment [6].
The importance of accurate early diagnosis to guide intervention decisions cannot be overemphasized. Behavioral metrics in children are notoriously difficult [7]. Some children display dramatic negative responses to maltreatment while for others the effects do not appear until later in life. Furthermore, some children transcend abuse to live healthier lives [8] while others grow up to become mentally ill, psychosocially maladjusted, plagued with chronic physical diseases, substance abuse and in some cases criminality [9,10].
It is unclear what the etiological pathways are from child maltreatment to negative or positive outcomes, and why not all children who experienced maltreatment are similarly affected. The identification of factors that account for variability in developmental outcomes of maltreated children is critical to informing theories of etiology in psychopathology and to guiding clinical intervention and preventive efforts for maltreated children. There have been calls for multiple levels of perspective in the investigation of the development of maltreated children [11,12], beyond the single focus on psychosocial predictors of adaptation among maltreated victims. In particular, recent literature on child maltreatment has begun to address the importance of incorporating more diverse biological assessments into the research on child maltreatment [13].
To make informed decisions about foster care, adoption, psychosocial, medical and other interventions, better objective data is needed. For example, beyond the effect of child maltreatment, a pre-adoptive risk factor, the family environment in the adopted youth’s new home may also influence psychosocial adjustments, both negatively and positively [14]. These factors could be monitored more effectively with biochemically-based objective clinical pathology markers. Relatively little attention has been paid to objective measures to distinguish these outcomes. Recently there has been growing attention to identify the biological impact of maltreatment in childhood. Intervention strategies currently primarily based on observation of behavior can be enhanced and refined with objective information from biological tests.
Objective tests most likely to be informative are: (1) chemical measurements of cortisol, a marker of chronic stress and a known correlate of poor outcomes in children; (2) Brain imaging, an emerging radiological diagnostic tool showing recent usefulness for psychiatric disorders; and (3) epigenetic markers in peripheral cells, such as buccal cells in saliva or white cells in blood. Recent studies in suicide risk and schizophrenia indicate a correlation of these disorders with DNA methylation at specific nucleotides [15,16].
Biomarkers and Types of Clinical Pathology Tests
Cortisol
Alterations in cortisol have been virtually uniformly identified in various forms of chronic stress, including early life maltreatment [17-21] and Post-Traumatic Stress Disorder (PTSD). Emerging consensus predicts that early life adversity may predispose to PTSD, possibly through cortisol-based and epigenetic mechanisms.
Cortisol has traditionally been measured in blood, saliva or urine by a variety of clinical pathology testing, including immunoassay and liquid chromatography tandem mass spectroscopy [19]. Natural diurnal fluctuations of cortisol in the body complicate interpretation of body fluid levels, particularly in subjects who are not on regular sleep-wake cycles. Recently, measurements of cortisol levels in hair have proven useful to determine long-term base line [26-29]. A centimeter of hair of half a pencil’s width, taken at the roots near the crown, has proven a valid and reproducible method to measure average serum cortisol levels for the previous month. Acquisition of hair has proven to be acceptable in multiple settings, including Latino children [30] and women in substance use disorder treatment centers [31]. Most clinical requests for cortisol measurements are related to ruling out endocrine diseases, such as Cushing’s. To date few clinical laboratories offer this testing service for psychiatric or public health purposes. The degree of difference between chronic-stress related cortisol alterations and normal diurnal rhythm is slight, requiring that the clinical laboratory test be sensitive, with careful attention to controls and narrow normal limits. Recently developed Eliza tests with colorimetric reaction dyes read in multi-well plate readers have proved sensitive enough. Tandem mass spec is also sensitive, although there are confounders with other steroids that co-elute with glucocorticoids.
Brain imaging
Functional magnetic resonance imaging (fMRI) has become a powerful tool for research into the effects of various disorders or experiences on brain connectivity. Much of these discoveries have not translated to the clinic as yet. While decreased volume in forebrain and hippocampus have been reported in adults who report experiencing early life adversity, this effect has not been validated in children at the time of the abuse. And a caveat for imaging studies is the possibility of head trauma in severe physical abuse. MRI is also prohibitively expensive for the low-income children who may suffer the most from childhood adversity and are the responsibility of the child welfare Services, who make decisions regarding placement and other interventions.
Other emerging biomarkers, epigenetic and DNA methylation
The persistent impact of childhood adversity on mental and physical health has led some to hypothesize that there must be a biochemical and/or anatomical irreversible event that an occur with abuse. The recent explosion of new data on epigenetic modifications of chromosomal DNA spurs investigation into this process as a target of abuse--and a biomarker for its potential long-term impact, as well as a biotarget for interventions aimed at reversing this impact.
Methylation of cytosines in DNA is one of the more easily measured epigenetic modifications (Figure 2). The NIH-funded ENCODE project [33], unleashed 24 primary reports in Nature and its affiliated journals in February 2015, with the posting of large amounts of sequencing data online. The Illumina HM450K beadchip platform identifies 450,000 pre-selected methylation sites in one run. NexGen sequencing of bisulfite-converted DNA theoretically produces information across every base in the genome. Bisulfite treatment converts un-methylated cytosines to thymidines, while protecting methylated cytosines (C), thereby altering the sequence of un-methylated DNA. The altered nucleotides are identified by comparison to a database human genome sequence, or to a nonbisulfite treated sequence from the same individual. These can either be detected for single gene of loci with PCR-based pyrosequencing, or across the genome with NexGen high throughput whole genome sequencing (Figure 2).
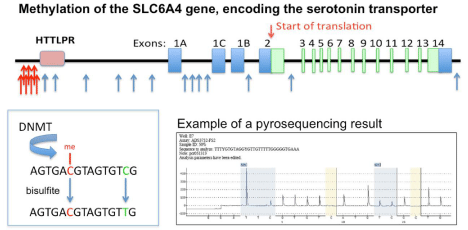
Figure 2: Methylation of DNA has been identified in a reasonably large study of self-reported cases of early childhood sexual abuse (top panel) [40]. The blue
arrows indicate these previously identified methylation sites. Additional methylation sites (red arrows) were found by Bearer lab in adults (unpublished observation).
The HTTLPR (pink) is a region of individual polymorphism and commonly contains either 14 (short) or 16 (long) numbers of tandem repeats of a 16-base pair
sequence. Levels of expression of this gene, which could be affected by DNA methylations, influence the function of the limbic emotional system in the brain. DNA
methylation is mediated by the DNA Methyl Transferases (DNMT) (lower left panel). Methylated cytosines can be detected because they are protected from bisulfite
conversion, which turns non-methylated Cs to Ts. Pyrosequencing, performed in molecular pathology clinical labs, identifies converted Cs (lower right panel). The
yellow highlight indicates un-methylated Cs which were all converted, and serves as an internal control of the conversion reaction. The blue highlights indicate
partially converted Cs in areas of suspected methylation, and the percent conversion is indicated above. This is a typical read-out from the Qiagen Pyro Mark Q24
pyrosequencing machine. Shown is a control sample in which the CGs were known to be 50% methylated. Thus 50% of the CGs should have converted to TG, and
none of the CN should be converted (ie protected) and all should have converted, as is shown.
The new sequence database from the ENCODE project and its software tools together with this emerging technology is exploding our understanding of the genome and its methylation, which no longer can be considered static. Some methylation sites seem to be quite dynamic, particularly in the brains of human children as they grow [34]. Methylation is a covalent chemical modification of DNA that may also be difficult, but not impossible, to reverse after critical periods in development. Hence epigenetic modification of the genome by DNA methylation is not necessarily a permanent event, and thus leaves more hope for therapeutic interventions than the gene therapy has offered which was complicated yet required to change the actual DNA sequence when a genomic mutation caused disease.
Methylated DNA was first reported as the mechanism by which the second X chromosome is silenced in women [35]. This discovery has lead to the current accepted notion that methylation, in general, down-regulates expression, particularly when the methylation occurs in promoter regions of genes. Thus increased methylation of a particular promoter would likely reduce the amount of that gene product in the cell. Indeed this turns out to be the case for some genes that have been intensively studied in oncology research. DNA methylation has turned out to be an important event in cancer growth, and many pathology laboratories now routinely screen for particular methylation sites. One such screen focuses on methylation of the promoter in the gene encoding Methyl Guanine Methyl Transferase (MGMT), initially found to occur in gliomas, primary brain tumors. Methylation of this region reduces expression of MGMT, a DNA repair enzyme. Giomas with this methylation pattern are more responsive to chemotherapeutic agents that alkylate DNA. Because this is a specific target, PCR-based pyrosequencing is used to identify methylation sites, a rapid and fairly inexpensive test compared to whole genome sequencing. If specific methylation sites are found and validated for adverse childhood experiences, similar less comprehensive, less expensive, targeted analysis will certainly be developed.
Some recent reports suggest that specific sites may be methylated in childhood maltreatment [37-42]. In humans, altered methylation of specific sites in the serotonin transporter gene (SLC6A4, also known as SERT or 5-HTT) occurs in young adult reporting sexual abuse in childhood [43]. If altered methylation influences expression of the SERT, this would have significant effects on the limbic system function in the brain, as we have shown for SERT knockout mice [44]. SERT is the target of cocaine and also for drugs used to treat depression. 5-HT agonists have also have been shown in animal studies to attenuate opiate withdrawal [45,46]. Hence regulation of SERT expression through epigenetic mechanisms by early life trauma could propagate to the adult and underlie the disabilities that correlate with childhood adversity, including emotional deregulation and substance abuse.
Yet how can we measure methylation occurring in the brain? Clearly brain biopsy is an uncomfortable option. Even sampling of cerebrospinal fluid, not expected to include neurons, but spinal tap seems too invasive. Recent evidence suggests that a small subset of genes whose methylation pattern is altered in the brain in a specific mental disorders, such as suicide, schizophrenia and autism, may also be altered in peripheral tissue, such as white blood cells or cells found in saliva [47]. Peripheral SLC6A4 (SERT) DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression [48], demonstrating that measurements of methylation in peripheral tissue is useful in diagnosing brain activity.
In a rat model of maternal care, epigenetic alterations (DNA methylation patterns) are different in pups from high versus low grooming mothers [49,50]. The gene for Brain-Derived Nerve Growth Factor (BDNF) [40] and for the glucocorticoid receptor [49]. It is only a matter of time before these genes also become targets for clinical pathology laboratory diagnostics.
Conclusion
Objective measurements to determine the degree and impact of childhood maltreatment and to monitor the effect of interventions are greatly needed. Clinical pathology stands at the forefront to develop assays for the practicing mental health provider and child services for social interventions. By coordinating testing with social workers at the frontline of family services, clinical pathology can make a huge impact on future generations. Childhood maltreatment is an emerging epidemic that needs pathologists to intervene for the protection of the future adults these abused children will become.
Acknowledgement
Special thanks to Kevin Reagan at UNM, and to Shawna Campbell and Diana Dimapindan at USC, for their administrative efforts. Supported by the Harvey Family Endowment (ELB), and R01MH087660 (ELB). RO1-HD39129 (FM and PT). We are grateful for pilot project funding from the UNM Clinical Translational Sciences Award, UL1 RR031977 and the USC Clinical Translational Sciences Award UL1 TR000130.
References
- Mennen FE, Kim K, Sang J, Trickett PK. Child neglect: definition and identification of youth's experiences in official reports of maltreatment. Child Abuse Negl. 2010; 34: 647-658.
- Heckman JJ. Role of income and family influence on child outcomes. Ann N Y Acad Sci. 2008; 1136: 307-323.
- De Wilde J, Soyez V, Broekaert E, Rosseel Y, Kaplan C, Larsson J. Problem severity profiles of substance abusing women in European Therapeutic Communities: influence of psychiatric problems. J Subst Abuse Treat. 2004; 26: 243-251.
- Herson M, Clinician; s Handbook of Child Behavioral Assessment 2006. San Diego, CA: Elsevier Academic Press.
- Trickett PK, Kurtz DA and P. K, Resilient outcomes in abused and neglected children: Based for strength-based interventions and prevention policies, in Investing in Children, Youth, Families and Communities: Strength-Based Research and Policy, Maton KI, et al. Editors. American Psychological Association: Washington, 2004; 73-95.
- Trickett PK, Noll JG, Putnam FW. The impact of sexual abuse on female development: lessons from a multigenerational, longitudinal research study. Dev Psychopathol. 2011; 23: 453-476.
- Trickett PK, Noll JG, Putnam FW. The impact of sexual abuse on female development: lessons from a multigenerational, longitudinal research study. Dev Psychopathol. 2011; 23: 453-476.
- Lynch M and Cicchetti D, An ecological-transactional analysis of children and contexts: the longitudinal interplay among child maltreatment, community violence and children's symptomatology. Dev Psychopathol, 1998;10: 235-257.
- Masten AS, Hubbard JJ, Gest SD, Tellegen A, Garmezy N, Ramirez M. Competence in the context of adversity: pathways to resilience and maladaptation from childhood to late adolescence. Dev Psychopathol. 1999; 11: 143-169.
- Cicchetti D and Rogosch FA, Gene x Environment interaction and resilience: effects of child maltreatment and serotonin, corticotropin releasing hormone, dopamine and oxytocin genes. Dev Psychopathol, 2012; 24: 411-427.
- Ji J, Brooks D, Barth RP, Kim H. Beyond preadoptive risk: The impact of adoptive family environment on adopted youth's psychosocial adjustment. Am J Orthopsychiatry. 2010; 80: 432-442.
- Guintivano J, Brown T, Newcomer A, Jones M, Cox O, Maher BS, Eaton WW. Identification and replication of a combined epigenetic and genetic biomarker predicting suicide and suicidal behaviors. Am J Psychiatry. 2014; 171: 1287-1296.
- Walton E, Hass J2, Liu J3, Roffman JL4, Bernardoni F5, Roessner V5, Kirsch M6. Correspondence of DNA Methylation Between Blood and Brain Tissue and its Application to Schizophrenia Research. Schizophr Bull. 2015;.
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Horm Behav. 2008; 53: 96-103.https://www.ncbi.nlm.nih.gov/pubmed/25154521
- Saxbe DE et al. Attenuated hypothalamic-pituitary-adrenal axis functioning predicts accelerated pubertal development in girls 1 year later. Dev Psychopathol, 2015; 27: 819-828.
- Trickett PK, Gordis E, Peckins MK, Susman EJ. Stress reactivity in maltreated and comparison male and female young adolescents. Child Maltreat. 2014; 19: 27-37.
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Dev Psychopathol. 2010; 22: 165-175.
- Ehlert U, Enduring psychobiological effects of childhood adversity. Psychoneuroendocrinology. 2013; 38: 1850-1857.
- Jones T, Moller MD. Implications of hypothalamic-pituitary-adrenal axis functioning in posttraumatic stress disorder. J Am Psychiatr Nurses Assoc. 2011; 17: 393-403.
- Yehuda R, Flory JD, Pratchett LC, Buxbaum J, Ising M, Holsboer F. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology (Berl). 2010; 212: 405-417.
- Radley JJ, Kabbaj M, Jacobson L, Heydendael W, Yehuda R, Herman JP. Stress risk factors and stress-related pathology: neuroplasticity, epigenetics and endophenotypes. Stress. 2011; 14: 481-497.
- Inder WJ, Dimeski G, Russell A. Measurement of salivary cortisol in 2012 - laboratory techniques and clinical indications. Clin Endocrinol (Oxf). 2012; 77: 645-651.
- Anna-Hernandez D, K.L, et al. Hair cortisol levels as a retrospective marker of hypothalamic–pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiology & behavior, 2011; 104: 348-353.
- Gow R, Thomson S, Rieder M, Van Uum S, Koren G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int. 2010; 196: 32-37.
- Wosu AC, Valdimarsdóttir U, Shields AE, Williams DR, Williams MA. Correlates of cortisol in human hair: implications for epidemiologic studies on health effects of chronic stress. Ann Epidemiol. 2013; 23: 797-811.
- Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, Newman C. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology. 2007; 92: 42-49.
- Woodruff SI, Conway TL, Edwards CC, Hovell MF. Acceptability and validity of hair collection from Latino children to assess exposure to environmental tobacco smoke. Nicotine Tob Res. 2003; 5: 375-385.
- Spear S, et al. “Just a little piece”: Acceptability of hair collection for a study on relapse prevention among women in substance use disorder treatment, 2015 submitted.
- Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Front Hum Neurosci. 2012; 6: 52.
- ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012; 489: 57-74.
- Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J. Global epigenomic reconfiguration during mammalian brain development. Science. 2013; 341: 1237905.
- Riggs AD, Pfeifer GP. X-chromosome inactivation and cell memory. Trends Genet. 1992; 8: 169-174.
- Hegi ME, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med, 2005; 352: 997-1003.
- Lutz PE, et al. Childhood maltreatment and stress-related psychopathology: the epigenetic memory hypothesis. Curr Pharm Des, 2015; 21: 1413-1417.
- Lutz PE, Turecki G2. DNA methylation and childhood maltreatment: from animal models to human studies. Neuroscience. 2014; 264: 142-156.
- Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, Rex-Haffner M. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2013; 110: 8302-8307.
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009; 65: 760-769.
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005; 57: 823-831.
- Schury K, Kolassa IT. Biological memory of childhood maltreatment: current knowledge and recommendations for future research. Ann N Y Acad Sci. 2012; 1262: 93-100.
- Vijayendran M, Beach SR, Plume JM, Brody GH, Philibert RA. Effects of genotype and child abuse on DNA methylation and gene expression at the serotonin transporter. Front Psychiatry. 2012; 3: 55.
- Bearer EL, Zhang X, Janvelyan D, Boulat B, Jacobs RE. Reward circuitry is perturbed in the absence of the serotonin transporter. Neuroimage. 2009; 46: 1091-1104.
- Dzoljic ED, Kaplan CD, Dzoljic MR. Effect of ibogaine on naloxone-precipitated withdrawal syndrome in chronic morphine-dependent rats. Arch Int Pharmacodyn Ther. 1988; 294: 64-70.
- Dzoljic MR, v Ent M, Kaplan CD, Saxena PR. Microinjection of 8-OH-DPAT into nucleus accumbens attenuates naloxone-induced morphine withdrawal syndrome. Prog Clin Biol Res. 1990; 328: 441-444.
- Bearer EL, DeLora MGA and Stephen JA, Comparative analysis of DNA methylation in saliva and brain of children with and without autism spectrum disorder (ASD), in Keystone Symposium on DNA Methylation 2015: Keystone, CO.
- Wang D, et al. Peripheral SLC6A4 DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression. PLoS One, 2012; 7.
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009; 12: 342-348.
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S. Epigenetic programming by maternal behavior. Nat Neurosci. 2004; 7: 847-854.