
Review Article
Austin J Clin Pathol. 2015; 2(3): 1036.
Gallbladder Cancer: Approaches to Biomarker Discovery
Tekcham DS and Tiwari PK*
Centre for Genomics, Molecular and Human Genetics, Jiwaji University, Gwalior, India
*Corresponding author: Tiwari PK, Centre for Genomics, Molecular and Human Genetics, Jiwaji University, Gwalior-474 011, Madhya Pradesh, India
Received: September 04, 2015; Accepted: October 29, 2015; Published: November 05, 2015
Abstract
Gallbladder cancer is a relatively uncommon global health issue, affecting most commonly the middle-aged women. Due to lack of early diagnostic marker and late detection of tumor, survival of GBC patients is compromised. Surgical resection of gallbladder is the only option for treatment. Here, we present the updates on molecular mechanism of GBC, current technological strategies, limitations and future prospects of biomarker discovery. The association of genetic, cytogenetic and epigenetic mechanisms with GBC has brought tremendous alteration in the expression of various protein coding genes. The recent advancements in high throughput technologies have replaced the conventional methods and now being considered as major tools for biomarker discovery. The earlier efforts on the identification of chromosomal loci harboring loss of heterozygosity alleles to use of both conventional and advanced technologies all, have identified some 4000 plus molecules from various biological sources of GBC (tissue, blood and cell lines). In this review, we have updated the list of molecules identified by major high throughput as well as conventional methods, which are currently the main targets in the discovery of diagnostic and or therapeutic biomarkers for GBC.
Keywords: Gallbladder; Cancer; Biomarker
Introduction
Gallbladder cancer is an uncommon cancer and is a female biased health issue, affecting the middle age group more often. In 2015, 10910 new GBC cases and 3700 estimated deaths were predicted in the United States [1]. The highest incidence of GBC is reported from Delhi, India (21.5/1,00,000), followed by South Karachi, Pakistan (13.8/1,00,000) and Quito, Ecuador (12.9/1,00,000) [2]. Our epidemiological study in north central Indian region showed significantly high incidence of GBC (7.8/1,00,000) [3]. The Indian Council of Medical Research (ICMR) Population Based Cancer Registry (ICMR-PBCR 2003-04) [4] reported the highest incidence of GBC in female to be 10.2 per lakh population, in Kamrup, Assam, India. Variations in the geographical distribution of the incidence of GBC reflect distinct ethnic (genetic) association. Gallstone disease is a potential risk factor of GBC. Increase in the number and size of the stones is directly related to the risk of developing GBC [5]. Reports have shown that 60% [6] to more than 80% GBC cases possess stone (multiple/single) [7]. Hundreds of genes have been identified to be significantly associated with GBC and having potential to be developed as useful diagnostic/ prognostic and even therapeutic biomarkers. Discovery of useful non-invasive biomarkers have always been desirable for diagnostic purposes, which are supposed to be safe and rapid [8]. Although our effort to dissect out the mechanism of gallbladder tumorigenesis is in progress, there are many challenges at various fronts. Hence, detailed investigations are needed in well classified group of samples, employing advance high through put technologies to understand the role of those genes, showing association with the GBC pathogenesis. This review is an attempt to summarize our current understanding on gallbladder tumorigenesis, use of technological strategies in search of clinically relevant biomarkers for early diagnosis, limitations and future prospects.
Mechanisms of gallbladder tumorigenesis
Various reports claimed Gall Stone Diseases (GSD) to be genetically modulated [9]. Upcoming information on family based investigations has replaced the quote as “GSD to be genetically associated” [10-11]. Formation of gallstone and Anomalous Pancreato-Biliary Duct Junction (APBDJ) are two pathological states for gallbladder tumor formation [12-14]. As GSD is an early pathological event, probably towards GBC, it is interesting to reveal its possible genetic association. We can call GSD as driver of GBC. Here, our effort is to present an update on the mechanisms that lead to GBC.
Genetic basis: Genetic association of GBC has become clear from various reports. Initially, the use of PCR based Loss of Heterozygosity (LOH) method could identify the loci, which harbor susceptibility genetic markers. Some of the earlier studies using PCR based allelotyping method detected more than 10 LOH-loci in GBC from Chile [15-16]. Restriction Fragment Length Polymorphism-PCR (RFLP-PCR) or gene sequencing is one of the most conventional methods used for identification of genetic variants [17-18]. Genomewide allelotyping identified 21 hot spot loci, including RNF4, SH3BP2, AF6q21, CD24, p73, DUTT1, FHIT, RAR-β, BLIMP1, CCNC, SMOH, PDGFR b-like, N33, FEZ1, p16Ink4/CDKN2, p15INK4b/CDKN2, NBCCS, DEC1, TSC1, CACNB2, MEN-1, TP53, STK11/LKB1 and NF2, distributed on 16 different chromosomes in GBC [19]. In a genome wide study in Japanese population, a genetic variant of Deleted in Colon Cancer (DCC) was found linked with Gallbladder Cancer [20]. Earlier studies claimed mutations to be responsible for their loss of expression of K-RAS [21], TP53 [22] and CDKN2A [15] in GBC, although later other mechanisms were also suggested. Hundreds of genes are expressed or regulated as genetic variants in GBC. Srivastava et al., (2011), in a meta-analysis, showed possible risk and association of various genetic variants with GBC [18]. Significant association of Prostate Stem Cell Antigen (PSCA) gene variants with GBC is reported in female patients in North Indian population [23]. In the same population group, increased risk of GBC was also found associated where genetic variants of Matrix Metalloproteinase (MMP- 2, 7, 9), tissue inhibitor of metalloproteinase (TIMP-2) [24], CYP1A1 and CYP1B1 [25] were pre-dominant. The ApoB-100 X+X+ genotype is also suggested to be associated with reduced risk of GBC [26]. Micro Satellite Instability (MSI), a common genetic mechanism of change in tandem repeats, is commonly observed in several genes, like TGFβR-II [27], BAT25, BAT26, D2S123 and D17S250 [28] in GBC. Loss of heterozygosity is one such mechanism suggested to play a key role in GBC [15,19,29] (Table 1).
SL. No.
No. of Molecules Identified
Significant markers
Biopsy
Technology
Status
References
LOH/Genes/SNP
Genomics
1
-------
KRAS, TP53, ERBB3, EGFR, ERBB2, ERBB4
Tissue
WES & UDS
GBC
[54]
2
14
IDH1, KRAS, NRAS, PIK3CA, MET
Tissue
SMA
GBC
[57]
3
26
TP53, STK11, RICTOR, TSC2
Tissue
NGS
GBC
[57]
4
------
D1S1597 and D1S407
Tissue
GWAS
GSD
[58]
5
130
DCC
Tissue
GWAS
GBC
[20]
6
21 hot spots
p73, DUTT1; FHIT, RARβ, RNF4; SH3BP2, AF6q21, CD24, BLIMP1, CCNC, TSC1, SMOH, PDGFR b-like, N33, p16, p15, NBCCS, DEC1, CACNB2, MEN-1, TP53, STK11/LKB1, NF2, FEZ1
Tissue
Genome-wide allelotyping
GBC
[19]
7
1281
RRM2, PTTG1, TYMS, CDC2, CCNB2, RACGAP1, SHMT2, ANK2, ACACB, MYOM1, ITGA7, CDKN1C ALDH1A2, CNN1, CES1, DES
Tissue
array CGH & RTPCR
GBC
[59]
8
5
APC, CDKN2A, ESR1, PGP9.5 and SSBP2
Tissue
ELISA based & qMSPCR GBC & GSD
GBC & GSD
[60]
Table 1: Selected list of LOH/genes/SNPs identified by high throughput technologies for biomarker identification in GBC and GSD.
Epigenetic basis: Introduction of methylation specific PCR to cancer research in 1996 opened the new area of “epigenetics” in cancer [30]. The large number of reports published so far has demonstrated epigenetics to be equally important as genetic mechanisms in the process of tumorigenesis. DNA methylation, histone modification (acetylation, phosphorylation, ubiquitination, sumoylation, methylation, etc.,), RNAi, etc., are a few examples of the epigenetic mechanisms. Much less is known about the role of epigenetics in GBC. Promoter hypermethylation of p16, p73, APC, MGMT, hMLH, RAR [31], SHP1, 3-OST-2, CDH13, p15, CDH1, RUNX3, RIZ1, p16INK4A and HPP1 genes [32] are commonly reported in GBC. We also screened the methylation pattern of several target tumor associated genes in the GBC patients of north central India, which showed APC [33] and PTEN (manuscript submitted) to be epigenetically down regulated in GBC. Among other genes analyzed, Maspin and 14-3-3 sigma genes also showed promoter methylation in most of the GBC cases [34]. Recently, Feng et al. demonstrated regulation of proliferation of GBC cells by histone acetyl transferase enzyme genes, ATF2, KAT5 and EPC1 [35]. A list of methylated genes in GBC may be found in Letelier et al [36]. The role of histone modification in GBC is still not fully known. Recent reports have implicated the role of small non-coding miRNAs, like miR-215-8p [37], miR-130a [38], miR-138 [39], miR-146b-5p [40], miR-26a [41], miR-1, miR-145 [42] and miR-20a [43] in GBC. More extensive investigation is required to understand fully the role of epigenetics in GBC (Table 2).
Sl. No.
No. of Molecules identified
Significant markers
Biopsy
Technology
Status
References
MicroRNAs
Transcriptomics
1
---
miR-26, miR-34a
Tissue/cell line
Validation Assays
GBC
[41,62]
2
---
miR-146b-5p
Tissue/cell lines
Validation Assay
GBC
[40]
3
11
let-7a, miR-21, miR-187, miR-143, miR- 202, and miR-335
Blood Serum
Micro Array
GBC
[56]
4
17
miR-210
Tissue
Illumina Sequencing & RTPCR
GBC
[63]
5
17/880
miR-20a
Tissue
miRNA Library Screening by High Content Screening
GBC
[64]
6
36/481
miR-1, miR-133, miR-143, miR-145
Tissue
Microarray & RTPCR
GBC
[42]
7
---
miR-21, miR-142-3p, miR-122, miR- 142-5p,miR-223
Mice Tissue
Microarray & RTPCR
GBC
[65]
Table 2: Selected list of small RNAs/ miRNAs identified by high throughput technologies for biomarker identification in GBC and GSD.
Alterations in Gene Expression in Gallbladder Cancer
The above mentioned epigenetic mechanisms alter the pattern of expression of many genes in GBC. The bile proteomic study by our group demonstrated more than 2500 proteins in human bile in cholecystitis [44]. Tan, et al., [45-46] also identified few dozens of proteins in GBC serum and tissue, and demonstrated Annexin A3, S100A10 and Haptoglobin as promising molecular targets of GBC. Increased expression of Phospho-mTOR [47], CLIC1 [48], ER1 and VEGF-A [49] and lower expression of Equilibrative Nucleoside Transporter member 1 (ENT1) [50] are suggested to lower the prognosis of GBC. Our recent proteome profiling of GBC tissues identified Prosaposin and Transgelin as potential diagnostic biomarkers for GBC [51]. SOX4 and HSPgp96 were recently reported to have independent prognostic values, strongly correlated with the survival of GBC patient [52-53]. Positive expression pattern of Maspin, IMP3, and S100P and the negative expression of pVHL are suggested to be useful in discriminating tumor tissues of GBC from normal ones [54] (see Table 3).
Sl. No.
No .of Molecules identified
Significant markers
Biopsy
Technology
Status
References
Proteins
Proteomic
1
2550
MARCKS, BSG, EPCAM, ICAM1, AXL, PAK1, ERBB2, NDRG1, CIAPIN1, ALCAM
Human bile
LC-MS/MS
Cholecystitis
[44]
2
544
S100A8
Tissue
2D & MS
GBC
[66]
3
495
S100A8
Tissue
2D & MS
Cholecystitis
[66]
4
24
S100A10, Haptoglobin, Cystatin-B, Profilin-1 and superoxide dismutase
Serum
MALDI-TOF-MS
GBC
[46]
5
46
Annexin A3, PEBP1
Tissue
2D & MS
GBC
[45,67]
6
26
CLIC1, Ezrin, Vimentin, Annexin A3, WD repeat domain 1, Triosephosphate isomerase, C1-tetrahydrofolate synthase, Rho GDP-dissociation inhibitor 1, T-complex protein 1, Heterogeneous nuclear ribonucleoprotein K, Glutamate dehydrogenase 1, Proteasome activator complex subunit 3 and Rab GDP-dissociation inhibitor beta
Cell line
2D & MS
GBC
[57]
7
6
ANXA4, HSP90 beta, Dync1h1, ACTA2, Serum albumin
Tissue
MALDI-TOF-MS
GBC
[68]
8
2575
NUCKS1, DMBT1, Mucin 5A, MUC13, LAMB3, PSAP, HMGB2, NEFH, S100AS, TAGL
Tissue
LC-MS/MS
GBC
[51]
9
26
Vimentin
Cell line/in vivo
2D & MS
GBC
[69]
Table 3: Selected list of proteins identified by high throughput technologies for biomarker identification in GBC and GSD.
Technological Strategies in Biomarker Identification
Advancement in the high throughput technologies has brought tremendous improvement in the ideological development to identify biomarkers during the last decade. Biomarker may be any molecule having prognostic, diagnostic and therapeutic properties. Although, conventional technologies are still useful for the purpose, the novel high throughput technologies have significantly added a number of significantly altered molecules to the list of biomolecules of diagnostic/ prognostic utility, already identified in GBC. Next generation sequencing, exome sequencing, RNA sequencing, Genome-Wide Association Study (GWAS), Proteomics, microarray, etc., are some of the major high throughput technologies currently being employed in the discovery of biomarkers in GBC (see Figure 1). Besides, selection of biopsy samples needs meticulous planning. Gallbladder tissues, bile, blood and urine are the main sources for the discovery of biomarkers, which may add to the specificity of the identified candidate genes. Exome sequencing based analysis have identified several mutations in TP53, KRAS and ERBB3. Extensive mutations were reported in the ErbB signaling pathways [55]. Similarly, a recent genome-wide association study in GBC cases from Japanese population identified DCC to be associated with GBC [20]. Our lab is currently validating the methylome data on GBC tissue samples obtained from Illumina’s Infinium 450K Beadchip array technology. So far, no published report is available on global methylation array and RNA seq in GBC. Microarray analysis of miRNA has updated the list of miRNAs in GBC and has drawn miRNA specific signatures in GBC [55]. Small RNAs, like miR-187, miR-143 and miR-202, are found to be key molecules having clinicopathological significance in GBC [56]. The other most promising strategy for biomarker identification could be the comparison of tissue, bile and serum proteomics together. Any altered molecule present in tissue may likely be released into body fluid during the course of tumorigenesis. Hence, simultaneous identification of molecules in all three media shall reveal the potential of differentially expressed molecules as a candidate biomarker in GBC. So far, no discovery has been made on the ultimate molecules with specificity and efficacy for diagnosis of GBC at an early stage. Identification of Annexin A3 [45], Prosaposin and Transgelin in tumor tissue [51], S100A10 and Haptoglobin in serum [46] and Chloride Intracellular Channel 1 (CLIC1) in GBC cell lines [48,57] are the part of this venture, however, having different prognostic values.
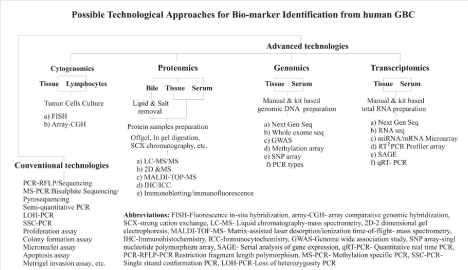
Figure 1: Flowchart showing various conventional and advanced technological based biomarker identification approaches available for GBC.
Limitations of strategies
There are certain common problems arise in such investigations. Availability of all types of biopsies is the main issue. Due to patient’s health issues, blood samples are often missed. Different technologies also have their own limitations. The whole genome or high through put technologies have higher chance of erroneous incorporation of some faulty data, which always needs validation by conventional technologies to confirm and correlate the findings. Early stages of the discovery, i.e., collection, preparation and processing of biological samples, are some of the important tasks to be taken care of in such investigations.
Future perspectives
The technological advancement always has a “to-go” approach to get more reliable and specific markers of GBC. Global methylation, miRNome and RNA sequencing analyses are the key technologies, which will have strong platforms to provide support and meaning to the currently available data obtained from proteomics and other methods in near future. We need to focus on all possible approaches to reach our goal. We have to wait till we identify molecules/markers with altered expression in tissue and blood (and hopefully, urine), and their subsequent confirmatory tests in other cancers too. Arriving at this stage may take some more time, but is not too far.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics CA: Cancer J Clin. 2015; 65: 5-29.
- Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009; 20: 146- 159.
- Barbhuiya MA, Singh TD, Poojary SS, Gupta S, Shrivastav BR, Tiwari PK. Gallbladder cancer incidence in Gwalior district of India: five year registry of a Regional Cancer Centre. Indian J Cancer. (In press).
- Van der Leeuw J, Visseren FL, Woodward M, Zoungas S, Kengne AP, Van der Graaf Y, et al. Predicting the effects of blood pressure-lowering treatment on major cardiovascular events for individual patients with type 2 diabetes mellitus: results from Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation. Hypertension. 2015; 65: 115-121.
- Vitetta L, Sali A, Little P, Mrazek L. Gallstones and gall bladder carcinoma. Aust N Z J Surg. 2000; 70: 667-673.
- Roa I, Araya JC, Wistuba I, Villaseca M, de Aretxabala X, Burgos L. [Gallbladder cancer in the IX Region of Chile. Impact of the anatomopathological study of 474 cases]. Rev Med Chil. 1994; 122: 1248-1256.
- Barbhuiya MA, Singh TD, Gupta S, Shrivastav BR, Tiwari PK. Incidence of gall bladder cancer in rural and semiurban population of north central India: A first insight. Internet J Epidemiol. 2009; 7: 4.
- Singh TD, Barbhuiya MA, Gupta S, Shrivastav BR, Jalaj V, Agrawal N, et al. Quantitative assessment of expression of lactate dehydrogenase and its isoforms 3 and 4 may serve as useful indicators of progression of gallbladder cancer: A pilot study. Indian J Clin Biochem. 2011; 26: 146-153.
- Zimmer V, Lammert F. Genetics in liver disease: new concepts. Curr Opin Gastroenterol. 2011; 27: 231-239.
- Attili AF, De Santis A, Attili F, Roda E, Festi D, Carulli N. Prevalence of gallstone disease in first-degree relatives of patients with cholelithiasis. World J Gastroenterol. 2005; 11: 6508-6511.
- Abu-Eshy SA, Mahfouz AA, Badr A, Gamal EMN, Al-Shehri MY, Salati M. Prevalence and risk factors of gallstone disease in a high altitude Saudi population. East Mediterr Health J. 2007; 13: 794-802.
- Chijiiwa K, Kozaki N, Naito T, Kameoka N, Tanaka M. Treatment of choice for choledocholithiasis in patients with acute obstructive supportive cholangitis and liver cirrhosis. Am J Surg. 1995; 170: 356-360.
- Hasumi A, Matsui H, Sugioka A, Uyama I, Komori Y, Fujita J, et al. Precancerous conditions of biliary tract cancer in patients with pancreaticobiliary maljunction: reappraisal of nationwide survey in Japan. J Hepatobiliary Pancreat Surg. 2000; 7: 551-555.
- Weiler H, Grandel A, Frühmorgen P. Congenital cystic dilatation of the cystic duct associated with an anomalous pancreaticobiliary ductal junction. Ultraschall Med. 2003; 24: 197-201.
- Wistuba II, Sugio K, Hung J, Kishimoto Y, Virmani AK, Roa I. Allele-specific mutations involved in the pathogenesis of endemic gallbladder carcinoma in Chile. Cancer Res. 1995; 55: 2511-2515.
- Wistuba II, Ashfaq R, Maitra A, Alvarez H, Riquelme E, Gazdar AF. Fragile histidine triad gene abnormalities in the pathogenesis of gallbladder carcinoma. Am J Pathol. 2002; 160: 2073-2079.
- Park SK, Andreotti G, Rashid A, Chen J, Rosenberg PS, Yu K, et al. Polymorphisms of estrogen receptors and risk of biliary tract cancers and gallstones: a population-based study in Shanghai. China. Carcinogenesis. 2010.
- Srivastava K, Srivastava A, Sharma KL, Mittal B. Candidate gene studies in gallbladder cancer: a systematic review and meta-analysis. Mutat Res. 2011; 728: 67-79.
- Wistuba II, Tang M, Maitra A, Alvarez H, Troncoso P, Pimentel F, et al. Genome-wide allelotyping analysis reveals multiple sites of allelic loss in gallbladder carcinoma. Cancer Res. 2001; 61: 3795-3800.
- Cha PC, Zembutsu H, Takahashi A, Kubo M, Kamatani N, Nakamura Y. A genome-wide association study identifies SNP in DCC is associated with gallbladder cancer in the Japanese population. J Hum Genet. 2012; 57: 235- 237.
- Tada M, Yokosuka O, Omata M, Ohto M, Isono K. Analysis of ras gene mutations in biliary and pancreatic tumors by polymerase chain reaction and direct sequencing. Cancer. 1990; 66: 930-935.
- Wistuba II, Gazdar AF, Roa I, Albores-Saavedra J. p53 protein over expression in gallbladder carcinoma and its precursor lesions: an immunohistochemical study. Hum Pathol. 1996; 27: 360-365.
- Rai R, Sharma KL, Misra S, Kumar A, Mittal B. PSCA gene variants (rs2294008 and rs2978974) confer increased susceptibility of gallbladder carcinoma in females. Gene. 2013; 530: 172-177.
- Sharma KL, Misra S, Kumar A, Mittal B. Higher risk of matrix metalloproteinase (MMP-2, 7, 9) and Tissue Inhibitor of Metalloproteinase (TIMP-2) genetic variants to gallbladder cancer. Liver Int. 2012; 32: 1278-1286.
- Sharma KL, Agarwal A, Misra S, Kumar A, Kumar V, Mittal B. Association of genetic variants of xenobiotic and estrogen metabolism pathway (CYP1A1 and CYP1B1) with gallbladder cancer susceptibility. Tumour Biol. 2014; 35: 5431-5439.
- Gong Y, Zhang L, Bie P, Wang H. Roles of ApoB-100 gene polymorphisms and the risks of gallstones and gallbladder cancer: a meta-analysis. PLoS One. 2013; 8: 614-656.
- Rashid A, Ueki T, Gao YT, Houlihan PS, Wallace C, Wang BS, et al. K-ras mutation, p53 over expression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clin Cancer Res. 2002; 8: 3156- 3163.
- Nagahashi M, Ajioka Y, Lang I, Szentirmay Z, Kasler M, Nakadaira H, et al. Genetic changes of p53, K-ras, and microsatellite instability in gallbladder carcinoma in high-incidence areas of Japan and Hungary. World J Gastroenterol. 2008; 14: 70-75.
- Chang HJ, Kim SW, Kim YT, Kim WH. Loss of heterozygosity in dysplasia and carcinoma of the gallbladder. Mod Pathol. 1999; 12: 763-769.
- Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylationspecific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996; 93: 9821-9826.
- House MG, Wistuba II, Argani P, Guo M, Schulick RD, Hruban RH, et al. Progression of gene hypermethylation in gallstone disease leading to gallbladder cancer. Ann Surg Oncol. 2003; 10: 882-889.
- Takahashi T, Shivapurkar N, Riquelme E, Shigematsu H, Reddy J, Suzuki M, et al. Aberrant promoter hypermethylation of multiple genes in gallbladder carcinoma and chronic cholecystitis. Clin Cancer Res. 2004; 10: 6126-6133.
- Singh TD, Poojary S, Bhunia S, Barbhuiya MA. Gupta S. Shrivastav BR, et al. Epigenetic regulation of APC in the molecular pathogenesis of gallbladder cancer. Indian J Med Res. (In press).
- Singh TD, Sharma P, Gupta R, Barbhuiya MA, Poojary S, Shrivastav BR, et al. Methylation patterns of MASPIN and STRATIFIN genes in Gall Bladder Cancer and Gall Stone Diseases. J Cancer Res Therapeut. 2012; 8: 1-46.
- Feng FL, Yu Y, Liu C, Zhang BH, Cheng QB, Li B, et al. KAT5 silencing induces apoptosis of GBC-SD cells through p38MAPK-mediated upregulation of cleaved Casp9. Int J Clin Exp Pathol. 2014; 7: 80-91.
- Letelier P, Brebi P, Tapia O, Roa JC. DNA promoter methylation as a diagnostic and therapeutic biomarker in gallbladder cancer. Clin Epigenetics. 2012; 4: 11.
- Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY, Gong W, et al. Long noncoding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015; 6: 1583.
- Ma MZ, Li CX, Zhang Y, Weng MZ, Zhang MD, Qin YY, et al. Long noncoding RNA HOTAIR, a c-Myc activated driver of malignancy, negatively regulates miRNA-130a in gallbladder cancer. Mol Cancer. 2014; 13: 156.
- Ma F, Zhang M, Gong W, Weng M, Quan Z. MiR-138 Suppresses Cell Proliferation by Targeting Bag-1 in Gallbladder Carcinoma. PLoS One. 2015; 10: 126-136.
- Cai J, Xu L, Cai Z, Wang J, Zhou B, Hu H. MicroRNA-146b-5p inhibits the growth of gallbladder carcinoma by targeting epidermal growth factor receptor. Mol Med Rep. 2015; 12: 1549-1555.
- Zhou H, Guo W, Zhao Y, Wang Y, Zha R, Ding J, et al. MicroRNA-26a acts as a tumor suppressor inhibiting gallbladder cancer cell proliferation by directly targeting HMGA. Int J Oncol. 2014; 44: 2050-2058.
- Letelier P, García P, Leal P, Álvarez H, Ili C, López J, et al. miR-1 and miR- 145 act as tumor suppressor microRNAs in gallbladder cancer. Int J Clin Exp Pathol. 2014; 7: 1849-1867.
- Chang Y, Liu C, Yang J, Liu G, Feng F, Tang J, et al. MiR-20a triggers metastasis of gallbladder carcinoma. J Hepatol. 2013; 59: 518-527.
- Barbhuiya MA, Sahasrabuddhe NA, Pinto SM, Muthusamy B, Singh TD, Nanjappa V, et al. Comprehensive proteomic analysis of human bile. Proteomics. 2011; 11: 4443-4453.
- Tan Y, Meng HP, Wu Q, Wang FQ, Wu HR. [Proteomic study of gallbladder cancer, with special reference on the expression and significance of annexin A3]. Zhonghua Bing Li Xue Za Zhi. 2010; 39: 382-386.
- Tan Y, Ma SY, Wang FQ, Meng HP, Mei C, Liu A, et al. Proteomic-based analysis for identification of potential serum biomarkers in gallbladder cancer. Oncol Rep. 2011; 26: 853-859.
- Leal P, García P, Sandoval A, Letelier P, Brebi P, Ili C, et al. Immunohistochemical expression of phospho-mTOR is associated with poor prognosis in patients with gallbladder adenocarcinoma. Arch Pathol Lab Med. 2013; 137: 552-557.
- Ding Q, Li M, Wu X, Zhang L, Wu W, Ding Q, et al. CLIC1 over expression is associated with poor prognosis in gallbladder cancer. Tumour Biol. 2015; 36: 193-198.
- Zhang LQ, Xu XS, Wan Y, Song SD, Wang RT, Chen W, et al. Prognostic implications of estrogen receptor 1 and vascular endothelial growth factor A expression in primary gallbladder carcinoma. World J Gastroenterol. 2015; 21: 1243.
- Espinoza JA, García P, Bizama C. Low expression of equilibrative nucleoside transporter 1 is associated with poor prognosis in chemotherapy-naïve gallbladder adenocarcinoma patients. Histopathology. 2015.
- Sahasrabuddhe NA, Barbhuiya MA, Bhunia S, Subbannayya T, Gowda H, Advani J, et al. Identification of prosaposin and transgelin as potential biomarkers for gallbladder cancer using quantitative proteomics. Biochem Biophys Res Comm. 2014; 446: 863-869.
- Wang C, Zhao H, Lu J, Yin J, Zang L, Song N, et al. Clinicopathological significance of SOX4 expression in primary gallbladder carcinoma. Diagn Pathol. 2012; 7: 41.
- Chen Y, Chen C, Ma C, Sun S, Zhang J, Sun Y. Expression of heat-shock protein in gallbladder cancer and its prognostic clinical significance. Int J Clin Exp Pathol. 2015; 8: 1946-1953.
- Shi J, Liu H, Wang HL, Prichard JW, Lin F. Diagnostic utility of von Hippel- Lindau gene product, maspin, IMP3, and S100P in adenocarcinoma of the gallbladder. Hum Pathol. 2013; 44: 503-511.
- Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z, et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet. 2014; 46: 872-876.
- Li G, Pu Y. MicroRNA signatures in total peripheral blood of gallbladder cancer patients. Tumour Biol. 2015.
- Wang JW, Peng SY, Li JT, Wang Y, Zhang ZP, Cheng Y, et al. Identification of metastasis-associated proteins involved in gallbladder carcinoma metastasis by proteomic analysis and functional exploration of chloride intracellular channel 1. Cancer Lett. 2009; 281: 71-81.
- Javle M, Rashid A, Churi C, Kar S, Zuo M, Eterovic AK, et al. Molecular characterization of gallbladder cancer using somatic mutation profiling. Hum Pathol. 2014; 45: 701-708.
- Puppala S, Dodd GD, Fowler S, Arya R, Schneider J, Farook VS. A genomewide search finds major susceptibility loci for gallbladder disease on chromosome 1 in Mexican Americans. Am J Hum Genet. 2006; 78: 377-392.
- Miller G, Socci ND, Dhall D, D’Angelica M, DeMatteo RP, Allen PJ, et al. Genome wide analysis and clinical correlation of chromosomal and transcriptional mutations in cancers of the biliary tract. J Exp Clin Cancer Res. 2009; 28: 62.
- Kagohara LT, Schussel JL, Subbannayya T, Sahasrabuddhe N, Lebron C, Brait M, et al. Global and gene-specific DNA methylation pattern discriminates cholecystitis from gallbladder cancer patients in Chile. Future Oncol. 2015; 11: 233-249.
- Jin K, Xiang Y, Tang J, Wu G, Li J, Xiao H, et al. miR-34 is associated with poor prognosis of patients with gallbladder cancer through regulating telomere length in tumor stem cells. Tumour Biol. 2014; 35: 1503-1510.
- Yang B, Liu B, Bi P, Wu T, Wang Q, Zhang J. An integrated analysis of differential miRNA and mRNA expressions in human gallstones. Mol Biosyst. 2015; 11: 1004-1011.
- Chang Y, Liu C, Yang J, Liu G, Feng F, Tang J, et al. MiR-20a triggers metastasis of gallbladder carcinoma. J Hepatol. 2013; 59: 518-527.
- Kitamura T, Connolly K, Ruffino L, Ajiki T, Lueckgen A, DiGiovanni J, et al. The therapeutic effect of histone deacetylase inhibitor PCI-24781 on gallbladder carcinoma in BK5.erbB2 mice. J Hepatol. 2012; 57: 84-91.
- Wang W, Ai KX, Yuan Z, Huang XY, Zhang HZ. Different expression of S100A8 in malignant and benign gallbladder diseases. Dig Dis Sci. 2013; 58: 150-162.
- Tan Y, Meng HP, Wang FQ, Cheng ZN, Wu Q, Wu HR. [Comparative proteomic analysis of human gallbladder carcinoma]. Zhonghua Zhong Liu Za Zhi. 2010; 32: 29-32.
- Huang HL, Yao HS, Wang Y, Wang WJ, Hu ZQ, Jin KZ. Proteomic identification of tumor biomarkers associated with primary gallbladder cancer. World J Gastroenterol. 2014; 20: 5511-5518.
- Dong P, He XW, Gu J, Wu WG, Li ML, Yang JH, Vimentin significantly promoted gallbladder carcinoma metastasis. Chin Med J (Engl). 2011; 124: 4236-4244.