
Case Report
Austin J Clin Pathol. 2015; 2(3): 1038.
Cyclin D1 Protein and CD5 Positive Diffuse Large B-Cell Lymphoma not Associated with CCND1 Gene Translocation
Katsushima H1,2*, Fukuhara N3, Kimura T4, Fujii M4, Scott SA5, Sasano H1 and Ichinohasama R2
1Department of Anatomic Pathology, Tohoku University Graduate School of Medicine, Japan
2Division of Hematopathology, Tohoku University Hospital, Japan
3Department of Hematology and Rheumatology, Tohoku University Graduate School of Medicine, Japan
4Division of Hematology/Oncology, KKR Suifu Hospital, Japan
5Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, USA
*Corresponding author: Hiroki Katsushima, Division of Hematopathology, Tohoku University Hospital, 1-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi, 980-8574, Japan
Received: April 28 , 2015; Accepted: December 01, 2015; Published: December 04, 2015
Abstract
Malignant lymphoma (ML) has a great deal of subtypes, and each subtype has each treatment strategy including the prognosis. The classification of subtypes largely depends on the pathological diagnosis, but we often have difficulty with the pathological diagnosis due to the various and complicated character of each subtype. The treatment strategy of diffuse large B-cell lymphoma (DLBCL) is different from that of mantle cell lymphoma (MCL). Therefore the differentiation of DLBCL and MCL requires scrupulous consideration in the case of ML especially composed of large lymphoid cells. In our case, neoplastic cells were medium to large size by the morphology, and it was difficult to distinguish DLBCL from pleomorphic variant of MCL. In the immunohistochemical staining, the cells were positive for cyclin D1, CD5 and Sox11, which was in accordance with the character of a variant MCL. However, fluorescence in situ hybridization (FISH) (FISH) demonstrated the absence of t(11;14)(q13;q32) ⁄ CCND1-IGH (CCND1 gene translocation), which prompted a diagnosis of atypical DLBCL. This is the first case report of CD5 positive DLBCL expressing cyclin D1 protein in the absence of CCND1 gene translocation.
Keywords: CD5; Cyclin D1; Diffuse large B-cell lymphoma; Fluorescence in situ hybridization; Mantle cell lymphoma
Introduction
Malignant Lymphoma (ML) has a great deal of subtypes, and each subtype has each treatment strategy including the prognosis. The classification of subtypes largely depends on the pathological diagnosis, but we often have difficulty with the pathological diagnosis due to the various and complicated character of each subtype. Mantle Cell Lymphoma (MCL) is a B-cell neoplasm composed of monomorphic medium-sized lymphoid cells. The immunohistochemistry of cyclin D1 is used for diagnostic purposes and helpful to distinguish MCL from other types of lymphomas, because cyclin D1 protein is aberrantly expressed in MCL as a result of t(11;14)(q13;q32) ⁄ CCND1-IGH (CCND1 gene translocation). Expression of CD5 is also highly characteristic, being detected in most of MCLs [1]. Diffuse large B-cell lymphoma (DLBCL) is a B-cell neoplasm composed of large lymphoid cells and a diffuse growth pattern [1]. DLBCL is typically negative for CD5 and not associated with CCND1 gene translocation.
In MCL, pleomorphic variant has been recognized. The variant generally has large sized cells, in contrast to typical classical variant MCL that is characterized by medium-sized cells. We often have a difficulty in distinguishing MCL variants from DLBCL. In this report we present a very unique case of CD5 positive DLBCL, expressing cyclin D1 protein in the absence of CCND1 gene translocation.
Case Presentation
An 80-year-old woman presented with decrease in appetite, denying fever, night sweats and weight loss. Physical examination indicated only slight splenomegaly and no lymphadenopathy. The infiltration of blastoid cells like matured lymphoid cells in peripheral blood and bone marrow aspiration were found. An excisional biopsy was not performed, but flow cytometry using bone marrow aspiration demonstrated that the blastoid cells were positive for CD19, CD20, CD5 and lamda-restricted surface immunoglobulin. Following the therapy of ML, she had received six cycles of immunochemotherapy with Rituximab + CHOP (Cyclophosphamide + Doxorubicine + Vincristine + Prednisolone) for 9 months and had completed remission.
Two and half years after the primary onset, she presented to have bilateral cervical lymphadenopathy, with the largest lymph node measuring 2cm in diameter at left and 3cm in diameter at right, not accompanied by fever, night sweats and weight loss. Slight splenomegaly was recognized, but no infiltration of lymphoid cells to bone marrow and no central nervous symptoms were found. The biopsy from left cervical lymph node was performed. Histologic examination, together with extensive phenotypic and molecular characterization, established the diagnosis of atypical DLBCL. She received immunochemotherapy with Rituximab + CHOP, but after one cycle she had a poor response. So she received three cycles of immunochemotherapy with Rituximab + DeVIC (Dexamethasone + Etoposide + Ifosfamide + Carboplatin), and had a complete response.
Three years and nine months after the primary onset, she had bilateral cervical adenopathy indicating a recurrence. More aggressive treatment was not attempted in accordance with her strong will, and after six months she died.
Histology and phenotype
An excisional biopsy revealed a lymph node with complete effacement of architecture due to proliferation of medium to large atypical cells with round, oval or elongated nuclei, one or more prominent nucleoli and moderately abundant cytoplasm (Figure 1A). There were numerous mitoses observed, scattered tingible body macrophages, and apoptotic cells. By flow cytometry, these cells were positive for CD5, CD7, CD19, CD20, CD22 and monoclonal lambda light-chain expression. By immunohistochemistry, they were positive for CD45, CD79a, CD5, CD7, CD20, CD22, Bcl-2, cyclin D1 and Sox11, but negative for CD3, CD10, Bcl-6 and MUM-1 (Figure 1B-1F). Staining for Ki-67 showed a high proliferation index (70%). Epstein Barr virus in situ hybridization analysis was not detected.
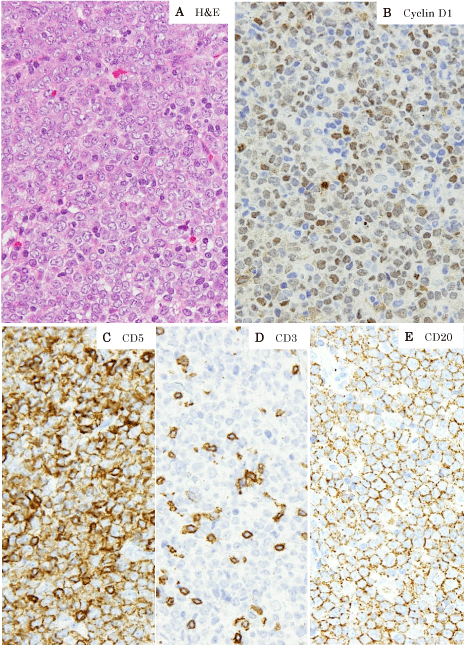
Figure 1: The lymphoma is composed of proliferation of medium to large
atypical cells with round, oval or elongated nuclei, one or more prominent
nucleoli and moderately abundant cytoplasm (A).
Immunohistochemistry performed on formalin-fixed paraffin-embedded
sections shows positivity for cyclin D1 (B), CD5 (C) and CD20 (E), in contrast
to negativity for CD3 (D).
Fluorescence in situ hybridization (FISH) and Immunoglobulin heavy chain (IGH) rearrangement analysis
FISH analysis was performed on 4μm paraffin-embedded sections, using the LSI CCND1 Dual Color Break Apart Rearrangement Probe (Vysis / Abbott). The result is that CCND1 gene translocation cannot be found (Figure 2). IGH gene rearrangements were detected.
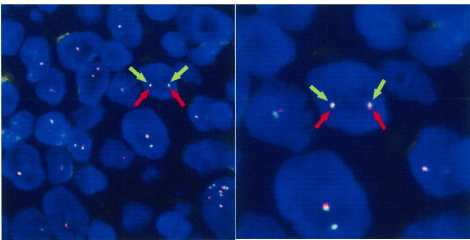
Figure 2: FISH analysis using probe for CCND1 shows no split signal, which
means that CCND1 gene translocation cannot be found.
Discussion
The cyclin D1 nuclear protein plays a major role in cell-cycle control, promoting transition from G1 to S phase and contributing to cell proliferation. Deregulation of cyclin D1 is known to play a key role in the pathogenesis of certain lymphomas [2]. With few exceptions, cyclin D1 immunohistochemical expression is observed in three lymphoma subtypes: MCL, plasma cell myeloma, and hairy cell leukemia [1]. Cyclin D1 protein is aberrantly expressed in most of MCL cases due to a recurring reciprocal translocation t(11;14) (q13;q32) involving CCND1 and IGH [3].
Most T cells and a subset of B cells express CD5 [4]. In mature B-cell neoplasms, CD5 expression is detected in the majority of patients with chronic lymphocytic leukemia and MCL, less frequently in DLBCL, and rarely in marginal zone B-cell and Burkitt lymphomas [5-9].
MCL is a B-cell neoplasm largely composed of monomorphic medium-sized lymphoid cells with irregular nuclear contours. These cells are most commonly positive for CD5, but negative for CD10 and BCL6. The pleomorphic variant has been recognized, which is aggressive subtype of MCL with large cell morphology and high proliferative activity. All cases are positive for BCL2 and almost all express cyclin D1 protein due to CCND1 gene translocation [1,3]. Additionally Sox11 expression is highly specific for MCL, as it is rarely expressed in other hematopoietic neoplasms [10-12].
DLBCL is a neoplasm of large B lymphoid cells with a nuclear size equal to or exceeding normal macrophage nuclei or more than twice that of normal lymphocytes. Lymph nodes demonstrate a diffuse proliferation of large lymphoid cells with total or partially effaced architecture. DLBCL requires additional analyses to exclude extramedullary leukaemias, Burkitt lymphoma and MCL pleomorphic variant. The neoplastic cells express pan B-cell markers such as CD19, CD20, CD22 and CD79a, but may lack one or more of these markers. The neoplastic cells express CD5 in 10% of DLBCL cases, which typically can be distinguished from MCL plemorphic variant by the absence of cyclin D1 protein expression [1].
In our case, based on the morphology and positive cyclin D1, CD5 and Sox11 immunohistochemical staining, the diagnosis was initially considered to be MCL with an unusual type such as pleomorphic variant. However, FISH demonstrated the absence of CCND1 gene translocation, which was not consistent with MCL. Based on the large cell lymphoma morphology, the possibility of an atypical DLBCL was considered.
Cyclin D1 protein expression in DLBCL is rare, with only few studies systematically examining the immunohistochemical detection of cyclin D1 in DLBCL [13]. For example, Zukerberg et al., and Bai et al., found no positive cases among 33 and 79 DLBCL cases, respectively [14,15]. Chang et al. reported one positive cyclin D1 protein case among their series of 21 DLBCL patients, but no genetic studies were performed in this study [16]. Ehinger et al., reported immunohistochemical detection of cyclin D1 in 10 of 231 (4%) DLBCLs, of which only one case carried CCND1 gene translocation [17]. Additionally, concerning cyclin D1-positive DLBCL without CCND1 gene translocation, Hsiao et al., reported 3 cases and Young et al., reported 30 cases [12,18]. These findings suggest the existence of a subset of DLBCL expressing cyclin D1 protein in the absence of CCND1 gene translocation, but in these studies all cases of cyclin D1 protein positive DLBCL were negative for CD5.
CD5 positive DLBCL is widely recognized and comprises about 10% of all DLBCLs. CD5 and cyclin D1protein positive DLBCLs have been reported, but the CCND1 gene translocation was not investigated [19]. Based on their description of cell morphology, these cases could potentially represent MCL pleomorphic variant.
Conclusion
The case described herein is the first case of CD5 positive DLBCL expressing cyclin D1 protein in the absence of CCND1 gene translocation. We insist that cyclin D1 and CD5 immunopositivity cannot distinguish all cases of MCL and DLBCL. Cyclin D1 protein expression may no longer be sufficient to distinguish pleomorphic MCL from DLBCL, regardless of CD5 status, and CCND1 gene translocation by FISH is necessary for a definitive diagnosis of MCL.
References
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press. 2008.
- Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G. Genes Dev. 1993; 7: 812-821.
- Lucioni M, Novara F, Riboni R, Fiandrino G, Nicola M, Kindl S, et al. CD5(-) diffuse large B-cell lymphoma with peculiar cyclin D1+ phenotype. Pathologic and molecular characterization of a single case. Hum Pathol. 2011; 42: 1204-1208.
- Kipps TJ. The CD5 B cell. Adv Immunol. 1989; 47: 117-185.
- Burns BF, Warnke RA, Doggett RS, Rouse RV. Expression of a T-cell antigen (Leu-1) by B-cell lymphomas. Am J Pathol. 1983; 113: 165-171.
- Ferry JA, Yang WI, Zukerberg LR, Wotherspoon AC, Arnold A, Harris NL. CD5+ extranodal marginal zone B-cell (MALT) lymphoma. A low grade neoplasm with a propensity for bone marrow involvement and relapse. Am J Clin Pathol. 1996; 105: 31-37.
- Ballesteros E, Osborne BM, Matsushima AY. CD5+ low-grade marginal zone B-cell lymphomas with localized presentation. Am J Surg Pathol. 1998; 22: 201-207.
- Lin CW, O'Brien S, Faber J, Manshouri T, Romaguera J, Huh YO, et al. De novo CD5+ Burkitt lymphoma/leukemia. Am J Clin Pathol. 1999; 112: 828-835.
- Yamaguchi M, Seto M, Okamoto M, Ichinohasama R, Nakamura N, Yoshino T, et al. De novo CD5+ diffuse large B-cell lymphoma: a clinicopathologic study of 109 patients. Blood. 2002; 99: 815-821.
- Mozos A, Royo C, Hartmann E, De Jong D, Baró C, Valera A, et al. SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1-negative subtype. Haematologica. 2009; 94: 1555-1562.
- Ek S, Dictor M, Jerkeman M, Jirström K, Borrebaeck CA. Nuclear expression of the non B-cell lineage Sox11 transcription factor identifies mantle cell lymphoma. Blood. 2008; 111: 800-805.
- Hsiao SC, Cortada IR, Colomo L, Ye H, Liu H, Kuo SY, et al. SOX11 is useful in differentiating cyclin D1-positive diffuse large B-cell lymphoma from mantle cell lymphoma. Histopathology. 2012; 61: 685-693.
- Rodriguez-Justo M, Huang Y, Ye H, Liu H, Chuang SS, Munson P, Prada-Puentes C. Cyclin D1-positive diffuse large B-cell lymphoma. See comment in PubMed Commons below Histopathology. 2008; 52: 900-903.
- Zukerberg LR, Yang WI, Arnold A, Harris NL. Cyclin D1 expression in non-Hodgkin's lymphomas. Detection by immunohistochemistry. Am J Clin Pathol. 1995; 103: 756-760.
- Bai M, Tsanou E, Agnantis NJ, Chaidos A, Dimou D, Skyrlas A, et al. Expression of cyclin D3 and cyclin E and identification of distinct clusters of proliferation and apoptosis in diffuse large B-cell lymphomas. Histol Histopathol. 2003; 18: 449-457.
- Chang CC, Liu YC, Cleveland RP, Perkins SL. Expression of c-Myc and p53 correlates with clinical outcome in diffuse large B-cell lymphomas. Am J Clin Pathol. 2000; 113: 512-518.
- Ehinger M, Linderoth J, Christensson B, Sander B, Cavallin-Ståhl E. A subset of CD5- diffuse large B-cell lymphomas expresses nuclear cyclin D1 with aberrations at the CCND1 locus. Am J Clin Pathol. 2008; 129: 630-638.
- Ok CY, Xu-Monette ZY, Tzankov A, O'Malley DP, Montes-Moreno S, Visco C, et al. Prevalence and clinical implications of cyclin D1 expression in diffuse large B-cell lymphoma (DLBCL) treated with immunochemotherapy: a report from the International DLBCL Rituximab-CHOP Consortium Program. Cancer. 2014; 120: 1818-1829.
- Zhang A, Ohshima K, Sato K, Kanda M, Suzumiya J, Kawasaki C, et al. Prognostic clinicopathologic factors, including immunologic expression in diffuse large B-cell lymphomas. Pathol Int. 1999; 49: 1043-1052.