
Special Article - Cancer Pathology
Austin J Clin Pathol. 2017; 4(2): 1049.
Histologic and Immunohistochemical Analysis and Study of Apoptosis in Three Cases of Histiocytic Sarcoma
Cristiano Claudino Oliveira*, Rafael Bispo Paschoalini, Maria Aparecida Custódio Domingues
Department of Clinical Pathology, São Paulo State University, Brazil
*Corresponding author: Claudino Oliveira C, Department of Pathology, Botucatu School of Medicine, São Paulo State University, Brazil
Received: April 06, 2017; Accepted: June 15, 2017; Published: June 22, 2017
Abstract
Histiocytic Sarcoma (HS) is a rare malignant neoplasia of hematopoietic origin and unknown etiology that is morphologically heterogeneous. HS has various clinical and epidemiological features that make this entity difficult to diagnose. We reported three HS cases with description of morphological pattern and immunohistochemical markers that helped the disease diagnosis in each case. We presented our experience with immunohistochemistry antibodies CD163, CD68, vimentin, lysozyme and S-100 in the diagnosis of this disease, together with 12 morphological criteria for its identification. Besides, we discussed about apoptosis marker in HS, something that may represent an investigative way to understand this diseases’ pathogenesis.
Keywords: Histiocytic sarcoma; Diagnosis; Immuno histochemistry
Introduction
Histiocytic Sarcoma (HS) is a malignant neoplasia characterized by the proliferation of cells with morphological and immunophenotypic features similar to mature tissue histiocytes [1]. It is a rare hematopoietic malignancy, comprising less than 1% non- Hodgkin lymphomas [2]. Its morphological heterogeneity overlaps with other malignancies, both lymphoid and no lymphoid, such as malignant melanoma, undifferentiated carcinomas, spindle cell sarcomas and anaplastic lymphomas, and is accompanied by various clinical and epidemiological features that make this entity difficult to diagnose [3]. It is uncommon and there are few series reported in the literature.
Its etiology is unknown [1]. There are reports of an association between HS and mediastinum germ cells tumors, particularly teratomas, with or without differentiation for gestational sac tumors. Teratocarcinoma cells may differentiate in vitro from hematopoietic lineages, which suggest that neoplastic histiocytic differentiation may originate in pluripotent embryonic cells [1,4,5]. HS can also precede or accompany Hodgkin Lymphoma, non-Hodgkin Lymphoma and Myelodysplasic Syndrome [1,6,7].
The use of immunohistochemical markers is necessary for a definitive diagnosis, however numerous markers are reported in the literature as auxiliaries in a definitive diagnosis, with no conclusions regarding which would be more specific [8,9]. The immunoexpression of the CD68, lysozyme, CD11c and CD14 markers must be positive; and myeloid lineage markers, as myeloperoxidase, CD33 and CD34 must be negative [3]. Currently, the use of the histiocytic marker CD163 has been proposed as more specific, which facilitates the neoplasia lineage determination [2,10].
About HS pathogenesis, there is a theory that indicates the possibility of compromised of apoptotic pathways, which may induce neoplastic histiocytic mortality and despite being well differentiated. These aspects are associated to a wide range of morphological features [11]. This theory would complement studies that determine morphological criteria and immunophenotypic markers. The definition of pathogenesis, associated with most frequent morphological patterns and specific immunomarkers, would assure appropriate diagnosis, efficient treatment and would improve disease prognosis in the future.
Here in, we studied three cases from our records with the objective of describe morphological and immunohistochemically aspects about these patients with HS, including an approach about apoptosis and cell proliferation markers.
Materials and Methods
We evaluated three confirmed cases from our service (Department of Pathology, Botucatu School of Medicine, São Paulo State University - FMB UNESP), which entered between 1998 and 2009, and were subject to morphological and immunohistochemical analysis.
Patients
Case 1: A 30 year-old man with a two-month history of painful cutaneous lesions on the left thorax. Additionally, he had intermittent fever, unmeasured weight loss and bilateral cervical/axillary adenomegaly. He had no relevant past medical history. Physical examination revealed an erythematous and infiltrative cutaneous lesion in the anterior chest wall and left cervical and supraclavicular enlarged lymph nodes measuring 2 cm at the largest diameter. Peripheral Blood Counts (PBC) revealed a hemoglobin level of 106 g/L (mean corpuscular volume 74 fL), platelets at 672 x 109/L, and WBC of 38 x 109/L with 31.5 absolute neutrophil counts. Lactate Dehydrogenase (LDH) was normal and albumin was 2.9g/dL. A biopsy of the cutaneous lesion and immunohistochemistry confirmed the diagnosis of histiocytic sarcoma. The patient was submitted to Compute Tomography (CT) scans of the chest, abdomen and pelvis, which showed homogeneous enlargement of spleen. The bone marrow trephine biopsy was normal and there were no other sites of extra nodal involvement.
Case 2: A 66 year-old woman had a four-month history of a nonpainful left cervical adenomegaly. She denied B-symptoms and, as a relevant past medical history, she had had a malignant melanoma, which was surgically removed 23 years before. A biopsy and immunohistochemical staining confirmed a diagnosis of histiocytic sarcoma. At that time, physical examination was normal and CT scans revealed no other sites of involvement. The bone marrow trephine biopsy was normal and PBC, LDH and albumin were all within normal ranges.
Case 3: The third case is not originally from our service. A 42 years-old woman presented with abdominal pain and a palpable mass in the small intestine. We do not have the patient’s follow-up due to the patient’s treatment was not realized in our service. Biopsy and immunohistochemistry study have confirmed the diagnosis of HS.
Morphology and Immunohistochemistry
For the morphological characterization of the HS cases, we selected the following criteria: cell size, chromatin, cell membrane, nucleolus, inclusion, cytoplasm, presence of bizarre cells, giant cells, Reed-Stenberg cells-like, rhabdoid cells, xanthomized cells, characterization of cohesivity, epithelioid features, fusiform features, inflammatory infiltrate, phagocytosis, apoptosis, mitosis, necrosis and hemosiderin.
To configure the immunohistochemistry panel, we have used 18 markers, including those considered most relevant according to current literature [8,11]. The immunohistochemical reaction was performed using Avidin Biotin Peroxidase [9] with antibodies anti: cytokeratin (AE1/AE3), EMA, Vimentin, S100 protein, HMB-45, Melan-A, Myeloperoxidase (MPO), CD21, CD1a, CD35, CD45, CD15, CD30, CD20, CD3, CD68, Lysozyme and CD163. Three markers for disease pathogeny were also tested: Fas Ligand, Bax and Caspase-3. The material was desparaffinized by endogen peroxidase block in hydrogen peroxide 3% incubation for 10 min at room temperature, protected from light. Next, heat antigenic reactivation was performed in citrate buffer (pH6.0/0.01mM) for 15 min. This step was performed in order to recover antigenic sites masked by formalin fixation and paraffin inclusion. For immunohistochemical characterization, the antibody-staining pattern was considered and classified as either diffuse or focal.
Results
In the first case, a 30-year-old male patient, the initial presentation was cutaneous, through an infiltrated erythematous plaque on the chest. In the morphological study, cell size was increased; proportional to the size of four lymphocytes, and the cells also presented the following criteria: vacuolated chromatin, irregular nuclear membrane, the presence of inclusion, vacuolated eosinophilic cytoplasm, few bizarre cells, the presence of cohesivity, epithelioid features, more exuberant inflammatory infiltration, mainly polymorphonuclear, phagocytosis, apoptosis and hemosiderin. The nucleolus were variable, a low number of “Reed-Sternberg-like” cells and rhabdoid cells were observed, giant and xanthomized cells were absent and fusiform appearance was also absent. A low number of mitotic figures and low necrosis were observed. The immunohistochemical study showed positive reaction for following markers: CD163, vimentin, CD68, CD45, CD30, lysozyme and Fas-ligand with diffuse distribution, and S-100 and caspase 3, which showed focal distribution. No significant staining was observed for the remaining markers (AE1/AE3, EMA, MELAN-A, CD20, CD3, CD15, MPO, CD21, CD35, CD1a, HMB45 and Bax), which were considered negative for this case.
This first patient received systemic chemotherapy with CHOP (cyclophosphamide 750mg/m2 on day 1, doxorubicin 50mg/m² on day 1, vincristine 1.4 mg/m² on day 1 and prednisone 100mg from day 1 to 5, every 21 days). He received eight cycles with complete resolution of the cutaneous lesions and disappearance of adenomegaly and splenomegaly. The patient remained in complete remission up to 9 months following treatment, when he ceased follow-up.
In the second case, the morphological study revealed the following results: large cells proportional to six lymphocytes, vacuolated chromatin, irregular nuclear membrane, conspicuous nucleoli, presence of inclusion, eosinophilic and vacuolated cytoplasm, more bizarre cells than case 1, giant cells and “Reed-Sternberg-like” cells were present and rhabdoid/xanthomized cells were absent. The cells presented the characteristics of cohesiveness, fusiform and epithelioid features, lymphocytic inflammatory infiltrate, increased phagocytic activity (lymphocytes and erythrocytes), mitosis and apoptosis, associated with greater presence of hemosiderin in the periphery and the absence of necrosis. The immunohistochemical study revealed diffuse staining for CD163, vimentin, CD68, and Fas-ligand lysozyme diffusely and focal staining for S-100 and caspase 3. No labeling was detected for CD45 and CD30, unlike our first case, nor for the remaining markers (AE1/AE3, EMA, Melan-A, CD20, CD3, CD15, MPO, CD21, CD35, CD1a, Bax and HMB45). This patient was submitted to chemotherapy with standard CHOP protocol for four cycles followed by consolidation involving field radiotherapy (36Gy total dose) with complete remission. The patient is still alive and asymptomatic six years after treatment.
In the third case, medium-sized cells were observed, approximately 4 lymphocytes in size like case 1, together with vacuolated chromatin, irregular nuclear membrane, variable nucleolus, presence of inclusion and eosinophilic and vacuolated cytoplasm, similar to the other cases. This case also presented bizarre cells, giant cells, “Reed-Sternberglike” and rhabdoid cells; however, unlike the other cases, xanthomized cells were present. Characteristics of cohesiveness, fusiform and epithelioid features, inflammatory infiltrate (lymphocytic and eosinophilic), phagocytosis (lymphocytes), apoptosis, mitosis and the presence of hemosiderin in the periphery were observed. In contrast, minimal necrosis was observed [12-15]. The immunohistochemical study of this case was also similar to case 2, with diffuse staining for CD163, vimentin, CD68, lysozyme and Fas-ligand, and focal staining for S-100 and caspase 3 and negative for the remaining markers, including the marker for apoptosis Bax.
Discussion
Twelve morphological criteria were simultaneously present in the three HS cases, which showed minimal variability, there were: medium to large cell size, vacuolated chromatin, irregular cell membrane, intranuclear inclusion, vacuolar eosinophilic cytoplasm, the presence of bizarre cells, cohesivity, epithelioid features, phagocytosis, stroma with inflammatory infiltrate, apoptosis and hemosiderin (Table 1 and Figure 1).
Case #1
Case #2
Case #3
Cellsize
Cells proportional to 4lymphocytes
Cells proportional to 6lymphocytes
Cells proportional to 4lymphocytes
Vacuolatedchromatin
Present
Present
Present
irregular nuclear membrane
Present
Present
Present
inclusion
Present
Present
Present
vacuolated eosinophilic cytoplasm
Present
Present
Present
bizarre cells
Present (few)
Present
Present
cohesivity
Present
Present
Present
epithelioid features
Present
Present
Present
inflammatory infiltration
Present (polymorphonuclear)
Present (lymphocytic)
Present (lymphocytic and eosinophilic)
phagocytosis
Present
Present
Present
apoptosis
Present
Present
Present
hemosiderin.
Present
Present
Present
nucleolus
Present (Variable)
Present
Present (Variable)
“Reed-Sternberg-like” cells
Present (lownumber)
Present
Present
rhabdoid cells
Present
Absent
Present
Giant cells
Absent
Present
Present
Xanthomized cells
Absent
Absent
Present
Fusiform appearance
Absent
Present
Present
mitotic figures
Present (lownumber)
Present
Present
necrosis
Present
Absent
Present (minimal)
Table 1: Comparative morphological aspects of the three cases.
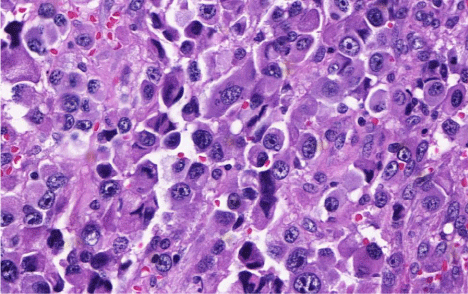
Figure 1: (H&E, 400x). Morphological aspect of HS. Note in this image the
cells in a medium to large size with vacuolar eosinophilic cytoplasm. There
are bizarre cells and epithelioid features.
Regarding the panel of immunohistochemical markers, five showed similar results in all three cases: CD163, CD68 (Figure 2), vimentin and lysozyme (Figure 3) all showed diffuse distribution, while S-100 distribution was focal. The markers CD45 and CD30 were positive strictly in the skin sample.
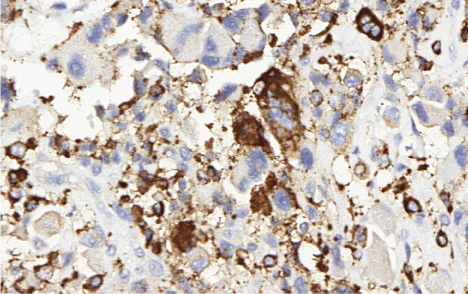
Figure 2: (CD68, 400x). The positivity for CD68 is an important factor for HS
diagnosis.
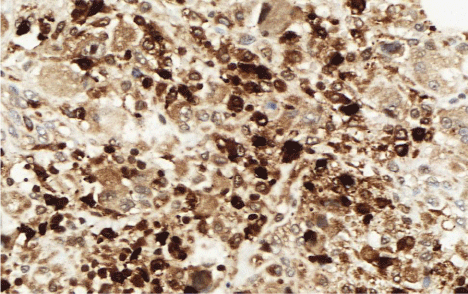
Figure 3: (Lysozyme, 400x). The combination of lysozyme with and CD68
and CD163 is considered the most important finding of immunohistochemistry
panel by literature.
The final immunohistochemical characterization of the three HS cases in which the initial presentations were distinct, is summarized in the (Table 2). Concerning markers for disease pathogeny, two showed positive results in all three cases: the apoptosis markers Fasligand (Figure 4) and caspase-3. The Bax marker was negative in the cases evaluated.
Positive (all)
Negative (all)
Variable
CD163 (diffuse)
AE1/AE3
CD45+ (only in skin)
CD68 (diffuse)
EMA
CD30+ (only in skin)
Vimentin (diffuse)
MELAN-A
Lysozyme (diffuse)
CD20
S-100 (focal)
CD3
Caspase (focal)
CD15
Fas-ligand (diffuse)
MPO
CD21
CD35
CD1a
HMB45
Bax
Table 2: Comparative immunohistochemical study of the three cases.
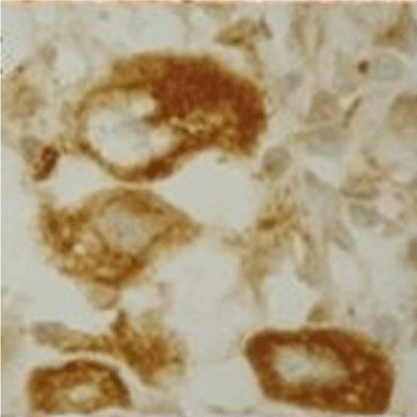
Figure 4: (Fas-ligand, 400x). There are diffuse patterns for Fas-ligand, as we
can observe in this image with detail in the neoplastic cell.
The Fas-ligand marker is an extrinsic factor in the apoptotic pathway and presented positive for all cases. Studies show the extrinsic Fas pathway is sufficient to induce apoptosis in some cell types. The literature reports that ligand expression in some tumors may be an evidence of a pro-inflammatory response [16]. Microenvironment alterations due to inflammatory processes can promote tumor growth and its immune response evasion.
Conclusion
We described three patients with HS, a rare neoplasm that represents a difficult challenge diagnosis for pathologists and hematologists. Our results are in line with the World Health Organization [1,17] that recommends using of CD163, CD68, vimentin, lysozyme and S-100 in the diagnosis of the disease. Furthermore, we observed 12 morphological findings in our patients that can be target of new and future studies, which will be very important for diagnosis criteria determination of HS. In addition, Fas-ligand marker analysis may suggest the occurrence of a pro-inflammatory state in our patients, but only three cases are insufficient for any conclusion. These findings must be known as a possible supporting for the hypothesis that HS pathogeny is related to an infectious process leading to microenvironment alterations that promote tumor growth.
References
- Swerdelow SH, Campo E, Harris ES. WHO Classification of tumours of haematopoietic and Lymphoid Tissues, WHO PRESS, International Agency for research on Cancer, Lyon. 2008.
- Vos JA, Abbondanzo SL, Barekman CL, Andriko JW, Miettinen M, Aguilera NS. Histiocytic sarcoma: a study of five cases including the histiocytic marker CD163. Modern Pathology. 2005; 18: 693-704.
- Pileri SA, Grogan TM, Harris NL. Tumours of histiocytes and accessory dendritic cells: An immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology. 2002; 41: 1-29.
- Suenaga M, Matsushita K, Kawamata N, Kukita T, Hamakawa Y, Gejima K, et al. True Malignant Histiocytosis with Trisomy 9 following Primary Mediastinal Germ Cell Tumor. Acta Haematologica. 2006; 116: 62-66.
- Cudenne CA, Johnson GR. Presence of multipotential hemopoietic cells in teratocarcinoma cultures. J Embryol Exp Morphol. 1981; 61: 51-59.
- Norbut AM, Foulis PR. Lymphoepithelioid cellular lymphoma (Lennert’s lymphoma) in association with malignant lymphoma, histiocytic type. Am J Clin Pathol. 1980; 73: 597-602.
- Sun W, Nordberg ML, Fowler MR. Histiocytic sarcoma involving the central nervous system: clinical, immunohistochemical, and molecular genetic studies of a case with review of the literature. Am J Surg Pathol. 2003; 27: 258-265.
- Mathé G, Gerard Marchant R, Texier JL, Schlumberger JR, Berumen L, Paintrand M. The two varieties of lymphoid tissue reticulosarcomas, histiocytic and histioblastic types. Br J Cancer. 1970; 24: 687-695.
- Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol. 2004; 122: 794-801.
- Wang E, Papalas J, Hutchinson CB, Kulbachi E, Huang Q, Sebastian S, et al. Sequential Development of Histiocytic Sarcoma and Diffuse Large B-cell Lymphoma in a Patient With a Remote History of Follicular Lymphoma With Genotypic Evidence of a Clonal Relationship: A Divergent (Bilineal) Neoplastic Transformation of an Indolent B-cell Lymphoma in a Single Individual. Am J Surg Pathol. 2011; 35: 457-463.
- Wakahashi K, Shimoyama M, Katayama Y, Minagawa K, Yoshida K, Sasaki R, et al. Histiocytic sarcoma with two immunohistopathologically distinct populations. Int J Hematol. 2010; 92: 642-646.
- Hornick JL, Jaffe ES, Fletcher ChDM. Extranodal histiocytic sarcoma. Clinicopathologic analysis of 14 cases of a rare epithelioid malignancy. Am J Surg Pathol. 2004; 28: 1133-1144.
- Copie BC, Wotherspoon AC, Norton AJ, Diss TC, Isaacson PG. True histiocytic lymphoma: A morphologic, immunohistochemical, and molecular genetic study of 13 cases. Am Journal Surg Pathol. 1998; 22: 1386-1392.
- Cao M, Eshoa C, Schultz C, Black J, Zu Y, Chang CC. Primary central nervous system histiocytic sarcoma with relapse to mediastinum: a case report and review of the literature. Arch Pathol Lab Med. 2007; 131: 301-305.
- Khosravi SP, del Castillo RA. Histiocytic sarcoma: a case report and review of the literature. An Med Intern. 2005; 22: 185-187.
- Whiteside TL. The role of death receptor ligands in shaping tumor microenvironment. Immun Invest. 2007; 36: 25-26.
- Oliveira CC, De Faveri J, Domingues MAC. Teratoma with Mediastinal Embryonic Carcinoma Concomiting Hystiocytic Sarcoma in the Bone Marrow. International Archives of Medicine. 2017; 10.