
Case Report
Austin J Clin Pathol. 2019; 6(1): 1059.
Primary Breast Neuroendocrine Carcinoma: Case Report of a Rare Entity
Kataria SP, Kumar S, Sadhu S*, Singh G and Sen R
Department of Pathology, Pt. B.D. Sharma Post Graduate Institute of Medical Sciences, Rohtak, Haryana, India
*Corresponding author: Sadhu S, Junior Resident, Department of Pathology, Pt. B.D. Sharma Post Graduate Institute of Medical Sciences, Rohtak, Haryana, India
Received: October 10, 2019; Accepted: November 07, 2019; Published: November 14, 2019
Abstract
Primary neuroendocrine carcinoma of breast is a rare malignancy and it has been reported to have an incidence varying from ‹0.1 to 5% of all breast carcinomas and ‹1% of all neuroendocrine tumours. The cells express neuroendocrine markers in more than 50% of the population. We report a case of a 72 year old female, who underwent fine needle aspiration for a lump in her breast and was diagnosed as primary neuroendocrine carcinoma of the breast based on cytological characteristics and specific immunohistochemical expression after cell block preparation.
Keywords: Breast carcinoma; Carcinoma, Neuroendocrine; Neuroendocrine cells
Introduction
The benign and malignant lesions of different organs may show neuroendocrine differentiation [1]. In a few recent studies, the incidence of foci of such a differentiation in breast carcinoma is reported to be 2-5% [2]. Breast Neuroendocrine Carcinoma or ”BNEC” was defined as a separate entity in 2003 by World Health Organization (WHO). Also, it was divided by WHO into three subtypes: solid neuroendocrine carcinoma, small/oat cell carcinoma and large cell neuroendocrine carcinoma [3]. Primary neuroendocrine carcinoma of breast is a rare malignancy and it has been reported to have an incidence varying from ‹0.1 to 5% of all breast carcinomas and ‹1% of all neuroendocrine tumours [4,5].
Primary neuroendocrine carcinoma of breast shows morphological features similar to neuroendocrine tumours of both the gastrointestinal tract and the lung. These are slow growing tumours and are derived from neuroendocrine cells. These express neuroendocrine markers in more than 50% of the cell population [3]. Here we report a case of primary neuroendocrine carcinoma of breast, based on subtle cytological characteristics and specific immunohistochemical expression as applied after cell block preparation.
Case Report
A 72-year-old female presented to the surgeon with the complaints of lump and pain in right breast for the past 3 months. On examination, a hard lump was felt in the retroareolar region of breast measuring 5 x 4 cm. Local skin temperature overlying the lump was raised. An examination of the axilla did not show any lymphadenopathy. The patient underwent fine needle aspiration for her breast lump which yielded blood mixed mucoid material as aspirate. The smears examined were hypercellular. Malignant cells were seen which appeared plasmacytoid at most places along with few round to polyhedral cells, both singly as well as in clusters with delicate cyanophilic cytoplasm and a relatively clean background. These cells revealed high nucleo-cytoplasmic ratio, round to ovoid nuclei with coarse granular chromatin (Figure 1). A cell block of the aspirate was also prepared (Figure 2) and immunohistochemistry was performed on the same. The tumour cells were found to be positive for CK (Figure 3), synaptophysin (Figure 4) and NSE (Figure 5) and negative for chromogranin and CD56. Based on cytomorphology and immunohistochemical markers, a diagnosis of primary breast neuroendocrine carcinoma was made.
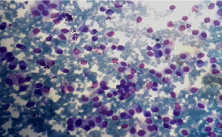
Figure 1a: Neuroendocrine Carcinoma of breast. A Hypercellular smear
showing malignant cells in clusters and lying singly. (Leishmann, x 100).
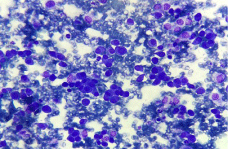
Figure 1b: Cells showing polygonal and plasmacytoid appearance (x 200).
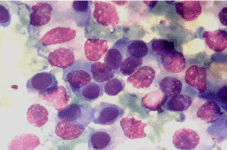
Figure 1c: Cells show pleomorphism, abundant cyanophilic cytoplasm and
coarse granular chromatin (Oil Emersion).

Figure 2: Cell block prepared from FNAC aspirate. H&E staining on high
power field showing neoplastic cells which are relatively uniform and small
in size and shape with salt and pepper chromatin seen in some cells and
moderate amount of granular eosinophilic cytoplasm. (H&E, x 400).
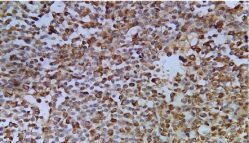
Figure 3: Neuroendocrine carcinoma. High power view showing cytokeratin
positivity (IHC, x 400).
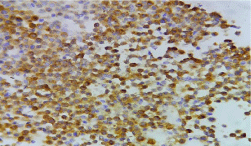
Figure 4: Showing synaptophysin positivity of tumour cells. (IHC, x 400).
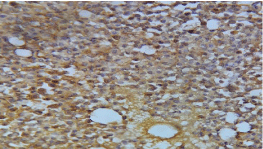
Figure 5: The breast tumor cells were reactive for neurone specific enolase.
(IHC, x 400).
Discussion
Neuroendocrine tumours are derived from neuroendocrine cells present throughout the body. These tumours are rare and are slow growing [6]. The overall prevalence has been estimated to be 1-2 cases per 1,00,000 persons [7]. Upalakalin et al in their study, searched the English literature, and found 59 cases of neuroendocrine tumors in the breast, 38 of which were primary breast neuroendocrine tumors. The mean age of these patients was found to be 66 years (range 35-97 years) and none had reported symptoms of carcinoid syndrome [8].
The existence of neuroendocrine cells in mammary tissue was indicated by Volger in 1947 [9]. Neuroendocrine differentiation in carcinoma breast is morphologically similar to its counterparts in the gastrointestinal tract, lung and other non-endocrine organs with endocrine differentiation [1]. The breast neuroendocrine carcinomas were earlier known as argyrophilic breast carcinoma, endocrine carcinoma or breast carcinoid. But, presently these are classified as breast carcinoma with neuroendocrine differentiation or primary neuroendocrine breast carcinoma. However, clinically these are indistinguishable from other types of breast tumours as per their presentation [3].
Neuroendocrine differentiation in breast carcinoma has been reported in 2-5% cases, most commonly in sixth to seventh decade of life in female patients [2]. It can be found in many breast histotypes, such as ductal, NOS, lobular [3,10], mucinous, tubular and papillary breast cancers [11]. Such a differentiation has also been shown to be present in male breast carcinoma [3].
The neuroendocrine tumours may represent either primary or metastatic lesions and the differentiation may be difficult even after microscopic examination. A component of ductal carcinoma in situ is the only absolute proof of the primary nature of breast carcinoma [12,13]. Depending upon the cell types, grade and degree of differentiation, neuroendocrine carcinoma has been categorized in the following subtypes: solid neuroendocrine carcinoma, small cell/ oat cell carcinoma and large cell neuroendocrine carcinoma [3].
As per the present consensus, the expression of neuroendocrine markers should be positive in more than 50% of the cell population for the diagnosis of breast neuroendocrine carcinoma. Also, this expression should be positive for at least two of the following markers: chromogranin A, synaptophysin, CD56 (NCAM) and NSE [3,5]. On ultra structural examination, demonstration of dense core granules or electron dense granules are characteristic [2]. In our case, immunohistochemical expression was seen in almost 100% of cells for NSE and synaptophysin, apart from cytokeratin. Thus, it was diagnosed as primary neuroendocrine carcinoma of breast.
References
- Ozbilim G, Kilicarslan B, Tezer E, Buyukkece A, Ustun M, Karaveli S, et al. Breast carcinoma showing neuroendocrine differentiation characterized with ectopic hormone production (2 case report). Turk J Med Sci. 2000; 30: 609- 613.
- Sapino A, Righi F, Cassoni P, Papotti M, Pietribiasi F, Bussolati G. Expression of the neuroendocrine phenomenon in carcinoma of the breast. Semin Diagn Pathol. 2000; 17: 127-137.
- Ellis IO, Schnitt SJ, Sastre-Garau X. Invasive breast carcinoma. In: Tavassoli FA, Devilee P, editors. World health organization classification of tumours. Pathology and genetics of the tumours of breast and female genital organs. Lyon: IARC press. 2003; 13-59.
- Ogawa H, Nishio A, Satake H, Naganawa S, Imai T, Sawaki M, et al. Neuroendocrine tumor in the breast. Radiat Med. 2008; 26: 28-32.
- Cai RX, Seng X. Clinical and pathological analysis of 13 cases of primary neuroendocrine carcinoma in breast. Tumour J World. 2008; 7: 268-270.
- Singh S, Aggarwal G, Kataria SP, Kalra R, Duhan A, Sen R. Primary neuroendocrine carcinoma of breast. J Cytol. 2011; 28: 91-92.
- Robertson RG, Geiger WJ, Davis NB. Carcinoid tumors. Am Fam Physician. 2006; 74: 429-434.
- Upalakalin JN, Collins LC, Tawa N, Parangi S. Carcinoid tumors in the breast. Am J Surg. 2006; 191: 799-805.
- Akhtar K, Zaheer S, Ahmad SS, Hassan MJ. Primary neuroendocrine carcinoma of the breast. Indian J Pathol Microbiol. 2009; 52: 71-73.
- Fetissof F, Dubois MP, Arbeille-Brassart B, Lansac J, Jobard P. Argyrophilic cells in mammary carcinoma. Hum Pathol. 1983; 14: 127-134.
- Ramos CV, Boesshart C, Restrepo GL. Intracystic papillary carcinoma of the male breast. Arch Pathol Lab Med. 1985; 109: 858-861.
- Fishman A, Kim HS, Girtanner RE, Kaplan AL. Solitary breast metastasis as first manifestation of ovarian carcinoid tumor. Gynecol Oncol. 1994; 54: 222-226.
- Bergholt T, Bruun E, Franzmann MB, Hvid-Jacobsen K, Henriksen FW. Carcinoid tumour of the breast. Eur J Surg Oncol. 1996; 22: 199-200.