
Case Report
Austin J Clin Pathol. 2020; 7(1): 1063.
Merkel Cell Carcinoma of the Right knee Treated with Somatostatin Analogue and Radiotherapy: a Case Report
Puliafito I1, Puglisi C2*, Falsaperla D1, Di Grazia A3, Colarossi C4, Forte S2, Giuffrida D1
1Department of Oncology, Mediterranean Institute of Oncology, Italy
2IOM Ricerca, Viagrande (CT), Italy
3Department of Radiotherapy REM, Viagrande (CT), Italy
4Department of Pathology, Mediterranean Institute of Oncology , Italy
*Corresponding author: Caterina Puglisi, IOM Ricerca, Via Penninazzo 11, Viagrande (CT), Italy
Received: June 08, 2020; Accepted: July 02, 2020; Published: July 09, 2020
Abstract
Introduction: Merkel Cell Carcinoma (MCC) is a rare and aggressive primary cutaneous neuroendocrine malignancy. Chemotherapy with cisplatin (CDDP) and etoposide (VP16) was the standard treatment option for metastatic MCC before the approval of the check point inhibitor Avelumab.
Aim: To describe a case of MCC located on the right knee in advanced stage, not eligible for therapy with CDDP and VP16 and treated with somatostatin analogues plus radiotherapy
Materials and Methods: A 76 year old man was referred to our center in March 2012 complaining a 8 cm symptomatic (painful and limitation of motion), vegetating and ulcerated lesion of the right knee. Biopsy of the lesion revealed Merkel cell carcinoma (ki 67 91%, CgA+, NSE+, CK 20–, CD 117– , TTF1-, SYN+); CT scan and Octreo Scan showed lesion in the right knee and homolateral inguinal lymph-node metastases. Because of HCV+ cirrhosis with thrombocytopenia (93.000/mm3), patient was ineligible to chemotherapy with CDDP and VP16, therefore he received somatostatin analogue plus radiotherapy.
Results: patient was treated with Lanreotide 120 mg every 28 days and radiotherapy on the primary lesion (20 Gy) and on the right inguinal lymph-nodes (20 Gy). The following examinations showed regression till disappearance of the primary and secondary lesions after about 7 months with durable complete response until today.
Conclusions: Somatostatin analogues plus radiotherapy represent a valid alternative treatment for patients with MCC not suitable to standard chemotherapy.
Keywords: Merkel cell carcinoma; Somatostatin analogues; Radiotherapy; Neuroendocrine tumor; Alternative therapy
Introduction
Merkel Cell Carcinoma (MCC) is a rare malignant tumor firstly described by Toker in 1972 as “Trabecular cutaneus carcinoma” [1]. Merkel cell carcinoma has different names: cutaneous apudoma, Merkel cell cutaneous neoplasm, small cell primary carcinoma of the skin, neuroendocrine carcinoma of the skin, primitive small cell carcinoma of the skin with endocrine differentiation, primary undifferentiated carcinoma of the skin, skin cell carcinoma of unknown obscure origin (Murky cell carcinoma) [2]. It is rare, with a poor prognosis and a low survival rate. It is characterized by the possible appearance of lymph nodes and by vascular involvement, this is due in both cases to a high rate of locoregional recurrence within the first year of tumor removal. The Surveillance of Rare Cancers in Europe (RARECARE) reported an incidence rate of 0.13 per 100.000 [3], while more recent data from United States of America report an incidence of 0.79 per 100.000 [4]. MCC is more frequent in people over 65 years old though there have been cases reported in young patients who were carriers of ectodermic congenital dysplasia syndrome [5]. A light skin type and solar exposure are considered risk factors for the disease as the tumor tends to develop in the body regions more exposed to sun: 50% in the head-neck region and 40% in the extremities. In nearly one third of all cases, this tumor is associated with other skin neoplasms such as basal cell or squamous cell carcinomas and Bowen carcinomas [6]. Merkel cell carcinoma may appear as a secondary malignancy in patients with immune disorders of different aetiologies: B-cell lymphoma, myeloma and chronic lymphocytic leukaemia; this tumor also appears in patients who have received organ transplants or are in protracted treatment with immunosuppressants [7]. Aggressiveness and mortality are even higher in such patients. Added risk factors was HIV infection.
The origin of this tumor is still unknown. In recent years a role in the carcinogenesis has been described for the virus called Merkel Cell Polyomavirus (MCV), that it seems to be involved in about 80% of the MCC [4]. Recent studies have discovered the presence of cytogenetic anomalies in different chromosomes [7]. Another study [8] found the presence of a mutation in the short arm of chromosome 10 in a great number of cases, it has demonstrated that this mutation would result in the inactivation of PTEN (tumor suppressor gene).
The tumor begins from neural crest derivative Merkel cell of epidermis situated in the basal layer which contains neuro-secretory granules. The primary lesion is often asymptomatic and may present as firm glassy, non-tender, bluish-red rapidly developing nodule at the time of appearance. Macroscopically the lesion usually appears as exophytic or plaque, with teleangectasias and ulcers, morphologically similar to basocellular and spinocellular carcinomas. Microscopically it appears as dermal-based lesion composed of monotonous small round cells with scanty cytoplasm expressing neuroendocrine immunohistochemical markers [9]. Currently, Merkel Cell Carcinoma is classified into three stages based on disease extension: localized disease, with regional lymph node metastases, and with distant metastases [10,11]. Surgery is the first treatment in patients without systemic diffusion, alternatively, chemotherapy based on cisplatin plus etoposide can be used to obtain a stabilization of disease for a median of 6-12 months [12,13].
In cases were the standard chemotherapy with carboplatin of cisplatin and etoposide (NCCN guidelines) was contraindicated, alternative therapy is used. There are two treatments for these cases. One option for alternative therapy is immunotherapy with Avelumab, that is a human PD-L1 inhibitor which blocks IgG1 lambda monoclonal antibody on the tumor cell, inhibiting the interaction between the PD-1 on T lymphocytes with the PD-L1 on the tumor cell. In 2017, Avelumab was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as a first-line treatment for patients with metastatic MCC.
The other option is chemotherapy based on inhibitors of Somatostatin receptor plus radiotherapy. Antitumor and antisecretory properties of somatostatin analogs in neuroendocrine tumors have been shown in numerous in vivo and in vitro studies. Somatostatin is a neuropeptide synthesized by paracrine cells. It has essential functions in monitoring paracrine, autocrine and endocrine roles. Besides the control of growth hormone production by the hypothalamic phase, somatostatin more over regulators many pancreatic, gastrointestinal and pituitary hormone secretion (eg, thyroid stimulating hormone, insulin, glucagon, gastric acid) [14]. Furthermore, somatostatin reduces intestinal motility and absorption, cell proliferation and vascular contractility. In addition, somatostatin is a neurotransmitter controlling locomotor activity and cognitive functions [14]. In this report we describe a case of MCC situated on the right knee in advanced stage, not eligible for therapy with CDDP and VP16 and treated (Before the Avelumab was approved) with Somatostatin analogues plus radiotherapy that presented until today a complete response
Case Report
We describe a case of a 76 years old man who referred to our center in March 2012 complaining a 8 cm vegetating and ulcerated lesion of the right knee. He was a known case of hypertension and he was a case of hyperglycemia. His past medical history included Acute Myocardial Infarction (AMI); implant of biventricular Internal Cardioverter Defibrillator (ICD); he has an aortic ectasia. Moreover he had a contributory family history for lung carcinoma and gastric tumor. At the time of admission he referred sacral and joint pains. On objective exam the lesion appeared irregular, and seemed to be symptomatic for pain and limitation of motility of the right knee (Figure 1).
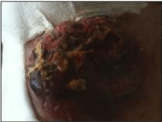
Figure 1: Knee lesion at the presentation.
To obtain precise characterization, a biopsy of the lesion was carried out revealing the presence of poorly differentiated neuroendocrine phenotype cells (ki 67 91%, CgA+, NSE+ , CK 20–, CD 117–, TTF1- , SYN+). On the basis of the histological findings a diagnosis of MCC was formulated. To achieve a complete evaluation of the disease extension, a Computed Tomography (CT) scan and laboratory tests were performed. The CT scan showed secondary right inguinal lymph nodes and also highlighted the signs of a chronic liver disease. The patient was affected by HCV+ cirrhosis with thrombocytopenia (93.000/mm3), elevated transaminases, and high levels of HCV-RNA. An Octreoscan was performed and the exam showed uptake of somatostatin receptors in the hip and in the right thigh, related with location of disease. The disease was classified as stage II disease (Disease with lymphatic spread) on the base of radiological examinations. Because of the cirrhotic disease and the thrombocytopenia, standard chemotherapy with Cisplatin plus Etoposide was contraindicated, for this reason, after specific informed consent has been acquired, an alternative therapy based on radiotherapy on the primary lesion (20 Gy) and on the right inguinal lymph-nodes (20 Gy) plus inhibitors of somatostatin receptor with Lanreotide 120 mg every 28 days has been administered. In August 2012, a clinical improvement of the right knee cutaneous lesion was evident (Figure 2), without progression of the disease at CT scan. Therapy with Lanreotide 120 mg every 28 days was then continued.
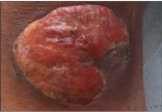
Figure 2: Knee lesion on August 2012.
In subsequent evaluations, performed every three months, the patient presented a progressive disease regression, in the absence of new lesions appreciable at CT and with a reduction of neoplastic markers; the lesion of the right knee continued to improve, and after about 7 months it was disappeared (Figure 3). To assess the response in February 2014 a PET with FDG was performed which results negative for disease with high metabolic index. The follow up examinations allowed therefore to define a complete response. At the time of writing, the patient is still in follow up with periodic control of tumor markers, physical and radiological exams; to date he does not show signs of disease progression.
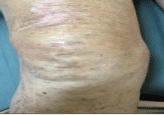
Figure 3: Knee lesion on March 2013.
Discussion and Conclusion
Merkel cell carcinoma is an aggressive tumor with poor prognosis [15]. It is seen as a painless, firm, solitary, red-purple colored small dermal nodule with a shiny surface and telengiectasias [16]. Mean age at diagnosis is 69. 50% of the cases are located in the head and neck region, 40% are located in the extremities and 10% are located at the trunk. The case shown reveals some peculiarities that would require further scientific investigations, in fact it is known the lack of studies and the poor general results obtained by the chemotherapeutic treatments of poorly differentiated neuroendocrine tumors (G3) that do not use the cisplatin + etoposide doublet [17,18]; in this case the stabilization of the disease was however obtained with a good local control (radiotherapy) associated with a “less aggressive” therapy based on somatostatin analogs.
Currently there are few cases in the literature of NET G3 treated with analogues SST, and most of them concerns combinations with chemotherapy for the control of symptoms in advanced GEP-NET [19]; the ability of such drugs to inhibit not only tumor endocrine secretion, but also the replicative tendency and activity of neoangiogenesisis supported by several studies [20]. Therefore, It is possible hypothesize the use of such drugs in patients who demonstrate an overexpression of the SSTR receptor on Octreoscan and that are not eligible for standard chemotherapy regimens; in our experience the control of the disease was excellent with no important toxicity. This case report suggests the possibility to use other treatment when standard therapy is not feasible.
Acknowledgments
patient was contacted and informed of this case report.
References
- Toker C. “Trabecular carcinoma of the skin” Arch Dermatol. 1972; 105: 107- 110.
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis, and clinical management. J Clin Oncol. 2009; 27: 4021-4026.
- Van der Zwan JM., Trama A, Otter R, Larranaga N, Tavilla A and Marcos Gragera R, et al. “Rare neuroendocrine tumours: results of the surveillance of rare cancers in Europe project” Eur J Cancer, 2013; 49: 2565-2578.
- Fitzgerald TL, Dennis S, Kachare SD, Vohra NA, Wong JH and Zervos EE. “Dramatic increase in the incidence and mortality from Merkel cell carcinoma in the United States” Am Surg. 2015; 81: 802-806.
- Miller RW and Rabkin CS. Merkel cell carcinoma and melanoma; etiological similarities and differences. Cancer Epidemiol Biomarkers Prev. 1999; 8: 153-158.
- Strokes JB, Graw KS, Dengel LT, Swenson BR, Bauer TW and Slingluff CL. Patients with Merkel cell carcinoma tumors < or = 1.0 cm in diameter are unlikely to harbor regional lymph node metastasis. J Clin Oncol. 2009; 10: 3772-3777.
- Mogha A, Fautrel A, Mouchet N, Guo N, Corre S and Adamski H. Merkel cell polyomavirus small T antigen mRNA level is increased following in vivo UVradiationPloS One. 2010; 5: 11423.
- Feng H, Shuda M, Chang Y and Moore PS. “Clonal Integration of a Polyomavirus in Human Merkel Cell Carcinoma”. Science. 2008; 319: 1096– 1100.
- Van Gele M, Leonard JH, Van Roy N, Cook AL and De Paepe A. Frecuent allelic loss at 10 q23 low incidence of PIEN mutations in Merkell cell carcinoma. Int J Cancer. 2001; 92: 409-413.
- Wong HH and Wang J. ”Merkel Cell Carcinoma” Archives of Pathology & Laboratory Medicine. 2010; 134: 1711-1716.
- Ruco L and Scarpa A. “Anatomia Patologica. Le basi”, Torino: Utet. 2007: 787-792.
- He W, Zhang D, Jiang J, Chen Y and Wu C. Merkel cell carcinoma in the left groin: a case report and review of the literature. Oncol Lett. 2015; 9: 1197–1200.
- Giuffrida D, Blanco G and Cardaci L, et al. “A Clinical Approach to Neuroendocrine Tumors”, Supportive and Palliative Cancer Care. 2006; 2: 17-19.
- Keskin O and Yalcin S. A review of the use of somatostatin analogs in oncology. Onco Targets Ther. 2013; 6: 471-483.
- Martino D, Blanco G, Mare M, Prestifilippo A, Vitale MP and Giuffrida D. “Chemotherapy in Neuroendocrine Tumours”, RivistaMedica. 2012; 1: 63-68.
- McAfee WJ, Morris CG, Mendenhall CM, Werming JW, Mendenhall NP and Mendenhall WM. Merkel cell carcinoma: treatment and outcome. Cancer. 2005; 104: 1761-1764.
- Beredjiklian B and Donthineni-Rao R. Tumors, Review of Hand Surgery. Saunders. 2004; 10: 189-206.
- Fjallskog ML, Granberg DP, Welin SL, Eriksson C, Oberg KE and Janson ET, et al. “Treatment with cisplatin and etoposide in patients with neuroendocrine tumors”. Cancer. 2001; 92: 1101-1107.
- Appetecchia M. and Baldelli R. “Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives”, J Exp Clin Cancer Res. 2010; 29: 19.
- Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P and Wied M, et al. “Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the effect of Octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group”. Journal of Clinical Oncology. 2009; 27: 4656-4663.