
Mini Review
J Clin Pathol. 2021; 8(1): 1070.
Concomitant Low-Grade Mucoepidermoid and Intraductal Carcinomas the Parotid Gland: Case Presentation with a Brief Review
Harada H1*, Goto S1, Kudo N2, Nakatsuka S3, Honma K4, Matsubara A2 and Kurose A1
1Department of Anatomic Pathology, Hirosaki University Graduate School of Medicine, Japan
2Department of Otorhinolaryngology, Hirosaki University Graduate School of Medicine, Japan
3Department of Diagnostic Pathology, Sakai City Medical Center, Japan
4Department of Diagnostic Pathology and Cytology, Okasa International Cancer Institute, Japan
*Corresponding author: Hiroshi Harada, Department of Anatomic Pathology, Hirosaki University Graduate School of Medicine, Visiting Research Fellow, 5 Zaifu, Hirosaki 036-8562, Japan
Received: February 11, 2021; Accepted: February 27, 2021 Published: March 08, 2021
Abstract
The patient was a Japanese female and a former thyroid cancer patient. A computed tomography scan indicated a small mass in the right parotid gland about 14 years after therapy for thyroid cancer. Pleomorphic adenoma was suspected by contrast-enhanced magnetic resonance imaging. Three years later, when she was 60 years old, fine needle aspiration cytology failed to determine a diagnosis and superficial parotidectomy was performed. There have been no signs of recurrence or metastasis for about 2 years thereafter. Histological examination revealed two histological types of low-grade mucoepidermoid carcinoma and low-grade intraductal carcinoma coexisting within the parotid mass. Although closely located, the two types of carcinoma had not intermingled. The mucoepidermoid carcinoma formed multilocular cystic structures, in which mucous, intermediate, and non-keratinizing squamous cells were intermingled. Exclusively predominant intraductal growth in the intraductal carcinoma showed a combination of cribriform and papillary-cystic patterns and diffuse positive reaction for S100 protein. Fluorescence in situ hybridization demonstrated the mucoepidermoid carcinoma had split signals for the MAML2 gene but no split signals were detected for the MAML2 and ETV6 genes in the intraductal carcinoma. We discuss the histomorphological features and diagnostic problems of the present case and the literature is briefly reviewed.
Keywords: Mucoepidermoid carcinoma; Intraductal carcinoma; Low-grade carcinoma; Multiple; Synchronous; Parotid gland
Abbreviation
CK: Cytokeratin; FISH: Fluorescence in Situ Hybridization
Introduction
Mucoepidermoid carcinoma is the most frequent carcinoma of the salivary glands, which is widely distributed and which can occur at any age. Most low-grade tumors consist of multilocular cystic structures containing mucous cells intermingled with intermediate cells and non-keratinizing squamous cells.
In contrast, low-grade intraductal carcinoma is still an underrecognized lesion, although rather known as low-grade salivary duct carcinoma termed by Delgado et al. in year of 1996 [1]. Brandwein et al. suggested the subsequent term low-grade cribriform cystadenocarcinoma [2], which was valid only in the 3rd edition of the WHO classification and which was then deleted in the current 4th edition. As the name implies, the tumor consists of mostly intraductal growth reminiscent of breast cancer, but its histomorphological and immunohistochemical features are different from those of ordinary salivary duct carcinoma closely resembling mammary invasive ductal carcinoma. Here, we describe an extremely rare and unique case in which both histological types coexisted to form a single mass in the unilateral parotid gland of a former thyroid cancer patient.
Case Presentation
Clinical summary
The patient was a Japanese female with a history of total thyroidectomy followed by cervical dissection and isotope treatment for thyroid cancer. A computed tomography scan indicated a small mass in the right parotid gland about 14 years later during long-term follow-up, which gradually increased in size. Pleomorphic adenoma was suspected on contrast-enhanced magnetic resonance imaging. Three years later, fine needle aspiration cytology was performed when she was aged 60 years, but sufficient sample for diagnosis was not collected and a superficial parotidectomy was performed. There have been no signs of recurrence or metastasis for about 2 years thereafter.
Pathological analysis
Macroscopically, the tumor was whitish and well-defined, but with an irregular-margined lobular mass, 12 mm in diameter. It was divided into four parts, three of which had similar lesions, and one with a different lesion (Figure 1 and 2a).
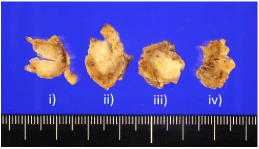
Figure 1: Macroscopically, the tumor was whitish and well-defined with an irregular margined lobular mass, 12 mm in diameter. It was divided into four slices.
i) to iv): Three of which had similar lesions, and one with a different lesion (unfortunately the cut surface is hidden in the other side).
Three parts of the tumor contained multilocular cystic structures consisting of columnar cells, non-keratinized squamous epithelium, and intermediate cells, all of which had mild cellular atypia (Figure 2b-2e). Mucous cells were scattered throughout and had intermingled together in the cystic walls (Figure 2f and g). The stroma contained mature lymphoid tissue with a germinal center and fibrous connective tissue.
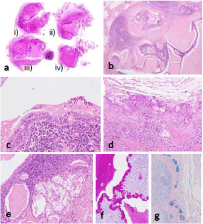
Figure 2: Similar to Figure 1, low-grade mucoepidermoid carcinoma corresponds to i) to iii), whereas low-grade intraductal carcinoma corresponds to iv) (a). The
low-grade mucoepidermoid carcinoma forms multilocular cystic structures with the stroma occupied by mature lymphoid tissue with a germinal center and fibrous
connective tissue (b). Cystic structures consist of columnar cells and mucous cells (c), including intermingled non-keratinized squamous cells and intermediate cells
(d). Focally invasive nests of clear cells are seen (e). Mucous cells are positive for PAS and Alcian blue stains (f and g).
The fourth part of the tumor contained a lesion consisting of a mixture of cystic and solid regions surrounded by thick capsular fibrous tissue connected to markedly hyalinized stroma (Figure 3a and b). The lesion consisted of ovoid and columnar cells with mild atypia, mixed with cribriform and intricately branched and anastomosing papillary structures (Figures 3c-3f). A small number of sebaceous-like cells containing many fine cytoplasmic vacuoles were also mixed in the tumor. Both histological types existed exclusively with no intermingling on any of the slices.
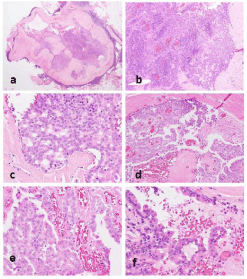
Figure 3: The low-grade intraductal carcinoma presents as a lesion with a mixture of cystic and solid regions, surrounded by a thick capsular fibrous tissue
connected with markedly hyalinized stroma (a and b). The lesion consists of ovoid and columnar cells with mild atypia, mixed with cribriform (c) and papillary
structures within the cystic space (d). Papillary fronds are intricately branched and anastomosing (e) and occasionally show a hobnail-like appearance (f).
Immunohistochemical analysis demonstrated both lesions were positive for various Cytokeratins (CKs) to varying degrees (Figures 4a-4c and 5a), and squamous and intermediate cells contained in the former were extensively positive for p63 (Figure 4d). The former was completely negative for S100 protein, while the latter showed a wide range of strong positive reactions (Figure 5b). Furthermore, in the latter, small or flat cells around the structure, which were not obvious on hematoxylin and eosin sections, were positive for smooth muscle actin, CK5/6, and p63 (Figure 5c-5e). In addition, mammaglobin was positive only in the latter, and no definite positive reaction for pSTAT5, DOG1, or androgen receptor was observed in either histological type.
Fluorescence in Situ Hybridization (FISH) were performed using the ZytoLight SPEC MAML2 Dual Color Break Apart Probe (ZytoVision, Bremerhaven, Germany) and Vysis LSI Dual Color Break Apart Rearrangement Probe (Abbott Molecular Inc, Des Plaines, USA) and revealed a definite split signal for the MAML2 gene in the former (Figure 4e), but not for the MAML2 or ETV6 genes in the latter. Based on these findings, the former lesion was determined to be a low-grade mucoepidermoid carcinoma and the latter a lowgrade intraductal carcinoma.
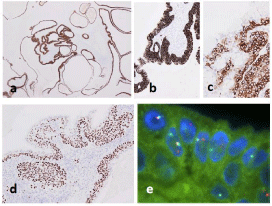
Figure 4: In the low-grade mucoepidermoid carcinoma, cystic structures lined by tumor cells, forming a layer of varying thickness, are positive for AE1/AE3 (a)
and CAM5.2 (b). Mucous cells lack immunoreactivity to CK5/6 (c). Squamous and intermediate cells are positive for p63 (d). FISH for the MAML2 gene shows a
definite split signal (e).
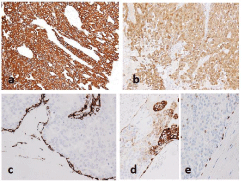
Figure 5: Tumor cells of low-grade intraductal carcinoma are diffusely positive for CAM5.2 (a) and S100 protein (b). Smooth muscle actin highlights residual
myoepithelial cells surrounding the histological structures (c) similarly to CK5/6 (d) and p63 (e). Also tumor cells focally positive for CK5/6.
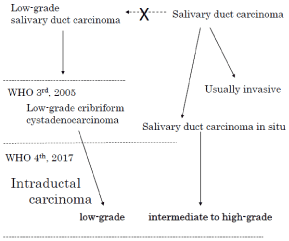
Schema: Correlation of the nomenclature.
Literature Review
Multiple salivary gland tumors
Previous studies of synchronous multiple salivary gland tumors reported that the Warthin tumor is the most common bilateral or unilateral multiple occurring tumor [3]. Furthermore, different histological types were reported in unilateral parotid glands, the most common combination being a Warthin tumor and pleomorphic adenoma [4]. A recent review collected 38 cases of benign and malignant tumors coexisting in the unilateral parotid gland, 22 of which were Warthin tumors and 11 were pleomorphic adenomas. Among malignant tumors, mucoepidermoid carcinoma was found in 11 cases and acinic cell carcinoma in 8 cases [5]. There are many points requiring verification including the potential for the malignant transformation of benign tumors and misidentifying partial images of malignant tumors as benign; therefore, a clear evaluation can be difficult if limited information is reported. In addition, the distinction between acinic cell carcinoma and secretory carcinoma is often unclear in older reports. Other reports show that basal cell adenoma [6-8] and oncocytoma [9,10] are benign tumors that frequently coexist with other histological types in the unilateral parotid gland, whereas sebaceous adenoma [10] and sebaceous lymphadenoma [7] are rarely observed.
Combinations of malignant tumors are even rarer, and a recent study reported a combination of secretory carcinoma and acinic cell carcinoma [11]. In that study, each formed an independent mass, the secretory carcinoma showed split signals by FISH for ETV6, and the acinic cell carcinoma contained distinctive acinic cells. The present case included mucoepidermoid carcinoma, a common malignant tumor, but to the best of our knowledge, no equivalent intraductal carcinoma including low-grade salivary duct carcinoma or low-grade cribriform cystadenocarcinoma was found in the descriptions of multiple salivary gland tumors.
Disease concept of intraductal carcinoma, its transition and related genetic mutations
Low-grade intraductal carcinoma (formerly known as low-grade salivary duct carcinoma), is immunohistochemically and biologically different from the original salivary duct carcinoma, and therefore was termed low-grade cribriform cystadenocarcinoma, a subtype of cystadenocarcinoma introduced in the revised 3rd edition of the WHO classification [2]. However, in the current 4th edition, it is integrated with lesions consisting of mainly intraductal growth and termed salivary duct carcinoma in situ [12], and became a lesion responsible for a low-grade spectrum of intraductal carcinomas (schema). It is characterized by cribriform and cystic structures with a papillary protrusion formed within the preexisting ducts and mixed in various proportions. Immunohistochemically it shows a broad and marked positive reaction for S100 protein, which is negative in ordinary salivary duct carcinoma. However, the description of immunohistochemical characteristics has been omitted from the 4th edition, so it is difficult to identify the lesion without understanding the background concerning the transition of the nomenclature mentioned above. This situation can cause misunderstanding as if lesions consisting of cells of the same lineage were subdivided into three grades, high, intermediate, and low, based on cytological atypia alone.
Even more confusingly, a recent study by Skalova et al. simplified the designation of low-grade ductal carcinoma to “intraductal carcinoma” and regarded as a distinctive entity [13]. This might cause further confusion, because it contradicts the description of the current WHO classification, which established the concept of disease by reintegrating the diseases, which were previously considered separate, even using a name that avoided describing the original characteristics of the constituent cells. If so then, tumors corresponding to intermediate- to high-grade should be clearly repositioned and termed “salivary duct carcinoma in situ” as reported by Simpson et al. [12] or “salivary duct carcinoma with a predominant intraductal component” following the nomenclature of breast cancer.
In addition, Skalova et al. described a case with invasive epithelial-myoepithelial carcinoma, which was included in a series reported by Weinreb et al. [14]. That case was considered a hybrid tumor that formed a single lesion, but neither study reported detailed histology or how epithelial-myoepithelial carcinoma with partial cystic dilatation was ruled out.
Currently, the most important differential diagnosis for low-grade ductal carcinoma is secretory carcinoma, which is capable of papillary cystic growth and expresses S100 protein [15]. Secretory carcinoma can also show intraductal growth, but its extent is very focal [16], which is the main difference compared with low-grade intraductal carcinoma [17]. Secretory carcinoma has become independent from conventional acinic cell carcinoma on the basis of having the same ETV6-NTRK3 fusion gene as secretory carcinoma of the breast [15]. However, recent studies reported ETV6 binds to diverse genes [17,18], and Skalova et al. reported a secretory carcinoma with a VIMRET fusion gene [20]; therefore, even if no split signal is observed for ETV6 by FISH, as in the present case, the possibility of secretory carcinoma cannot be ruled out by that point alone.
Indeed, considering a tumor that does not have the ETV6- NTRK3 fusion gene as a secretory carcinoma is nothing but allowing the diagnosis of a secretory carcinoma to be made by disregarding the fusion gene and it will be necessary to determine precisely the histomorphological and immunohistochemical characteristics of tumors. In that respect, pSTAT5, which is considered to be the best marker in terms of both sensitivity and specificity, should be regarded as more useful [21,22]. In that respect, pSTAT5, considered the best marker for secretory carcinoma in terms of both sensitivity and specificity, should be more useful [21,22]. In the present case, pSTAT5 was negative. Although the RET part of the fusion gene is common to the fusion gene NCOA4-RET and TRIM27-RET found in low-grade intraductal carcinoma [13,14], the relationship between low-grade intraductal carcinoma and secretory carcinoma is unclear at present.
Unique features and interpretation of the present lesion
In the present case, areas other than low-grade intraductal carcinoma, previously a variant of cystadenocarcinoma, were initially regarded as cystadenoma, and the possibility that intraductal carcinoma developed due to the malignant transformation of cystadenoma was considered. However, this carcinoma was confirmed as mucoepidermoid carcinoma because split signals were observed for MAML2 by FISH, a useful tool to confirm the diagnosis of low-grade mucoepidermoid carcinoma [23]. Because mucoepidermoid carcinoma was confirmed in the present case, we considered the prognosis should be determined by mucoepidermoid carcinoma, a purely invasive carcinoma even though it is low-grade malignant. Although rare, low-grade intraductal carcinoma can show lymph node metastasis as a result of extremely localized invasion [24]; therefore, is no clear difference in prognosis between the two carcinomas.
The present case resembled previously-reported hybrid tumors, in that multiple histological types were present in a single mass [25]; however, in our case there was no evidence of intermingled histological types in the mass. It is presumed that hybrid tumors have the same origin, although they show different histology, but in the present case, the intraductal carcinoma did not share the rearrangement of MAML2 found in the mucoepidermoid carcinoma. Furthermore, the histology indicated no mixing of cell types suggesting this was not a collision tumor. Although the two tumors were very close to each other, they developed independently and formed a single mass due to the surrounding fibrosis.
Interestingly, cases of multiple salivary gland tumors may be associated with extrasalivary gland tumors, of which thyroid cancer and breast cancer are examples [25]. The present case had a history of treatment for thyroid cancer, and this might form part of a common etiology for these three types of tumors.
Conclusion
We reported a unique case of a small mass occurring in the parotid gland of a former thyroid cancer patient, in which low-grade mucoepidermoid carcinoma and low-grade intraductal carcinoma coexisted. The lesion is unique because it is neither a hybrid tumor nor a collision tumor on the basis of the clear localization of each tumor. In addition, considering the similarity between lowgrade salivary carcinomas such as mucoepidermoid carcinoma, cystadenocarcinoma, secretory carcinoma, and low-grade intraductal carcinoma, the present case is interesting in terms of their relationship. This is the first report of a low-grade intraductal carcinoma included in synchronous multiple salivary gland tumors occurring in the ipsilateral parotid gland.
References
- Delgado R, Klimstra D, Albores-Saavedra J. Low grade salivary duct carcinoma. A distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer. 1996; 78: 958-967.
- Brandwein-Gensler M, Hille J, Wang BY, Urken M, Gordon R, Wang LJ, et al. Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol. 2004; 28: 1040-1044.
- Gnepp DR, Schroeder W, Heffner D. Synchronous tumors arising in a single major salivary gland. Cancer. 1989; 63: 1219-1224.
- Herce-Lopez J, Salazar-Fernandez CI, Mayorga-Jimenez F, Gallana-Alvarez S, Perez-Sanchez JM. Synchronous unilateral parotid neoplasms. A case report. Med Oral Patol Oral Cir Bucal. 2009; 14: E90-E92.
- Ochal-Choinska A, Bruzgielewicz A, Osuch-Wojcikiewicz E. Synchronous multiple unilateral parotid gland tumors of benign and malignant histological types: case report and literature review. Braz J Otorhinolaryngol. 2019; 85: 388-392.
- Goh PM, Cheah E. Synchronous tumours of the parotid gland with different histology. Br J Oral Maxillofac Surg. 1989; 27: 198-202.
- Scheller EL, Pritchett CV, Shukla A, Pepper JP, Marentette LJ, McHugh JB. Synchronous ipsilateral sebaceous lymphadenoma and membranous basal cell adenoma of the parotid. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013; 115: e41-e46.
- Gvozdjan K, Groth JV, Patel TN, Guzman G, David O, Cabay RJ. Synchronous ipsilateral parotid tumors with cytologic-histologic correlation. Head Neck Pathol. 2016; 10: 265-268.
- Araki Y, Sakaguchi R. Synchronous oncocytoma and Warthin’s tumor in the ipsilateral parotid gland. Auris Nasus Larynx. 2004; 31: 73-78.
- Bab IA, Ulmansky M. Simultaneously occurring salivary gland tumors of different types. J Oral Surg. 1979; 37: 826-828.
- Mossinelli C, Pigni C, Sovardi F, Occhini A, Preda L, Benazzo M, et al. Synchronous parotid (mammary analog) secretory carcinoma and acinic cell carcinoma: report of a case. Head Neck Pathol. 2019; 13: 686-691.
- Simpson RH, Desai S, Di Palma S. Salivary duct carcinoma in situ of the parotid gland. Histopathology. 2008; 53: 416-425.
- Skalova A, Vanecek T, Uro-Coste E, Bishop JA, Weinreb I, Thompson LDR, et al. Molecular profiling of salivary gland intraductal carcinoma revealed a subset of tumors harboring NCOA4-RET and novel TRIM27-RET fusions: A report of 17 cases. Am J Surg Pathol. 2018; 42: 1445-1455.
- Weinreb I, Bishop JA, Chiosea SI, Seethala RR, Perez-Ordonez B, Zhang L, et al. Recurrent RET gene rearrangements in intraductal carcinomas of salivary gland. Am J Surg Pathol. 2018; 42: 442-452.
- Skalova A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010; 34: 599-608.
- Connor A, Perez-Ordonez B, Shago M, Skalova A, Weinreb I. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol. 2012; 36: 27-34.
- Skalova A. Mammary analogue secretory carcinoma of salivary gland origin: an update and expanded morphologic and immunohistochemical spectrum of recently described entity. Head Neck Pathol. 2013; 7: S30-S36.
- Ito Y, Ishibashi K, Masaki A, Fujii K, Fujiyoshi Y, Hattori H, et al. Mammary analogue secretory carcinoma of salivary glands: a clinicopathologic and molecular study including 2 cases harboring ETV6-X fusion. Am J Surg Pathol. 2015; 39: 602-610.
- Skalova A, Vanecek T, Simpson RH, Laco J, Majewska H, Baneckova M, et al. Mammary analogue secretory carcinoma of salivary glands: molecular analysis of 25 ETV6 gene rearranged tumors with lack of detection of classical ETV6-NTRK3 fusion transcript by standard RT-PCR: Report of 4 cases harboring ETV6-X gene fusion. Am J Surg Pathol. 2016; 40: 3-13.
- Skalova A, Baneckova M, Thompson LDR, Ptakova N, Stevens TM, Brcic L, et al. Expanding the molecular spectrum of secretory carcinoma of salivary glands with a novel VIM-RET fusion. Am J Surg Pathol. 2020; 44: 1295-1307.
- Kawahara A, Taira T, Abe H, Takase Y, Kurita T, Sadashima E, et al. Diagnostic utility of phosphorylated signal transducer and activator of transcription 5 immunostaining in the diagnosis of mammary analogue secretory carcinoma of the salivary gland: A comparative study of salivary gland cancers. Cancer Cytopathol. 2015; 123: 603-611.
- Hamamoto Y, Harada H, Kohara M, Honma K, Nakatsuka SI, Morii E. Usefulness of immunohistochemistry to distinguish between secretory carcinoma and acinic cell carcinoma in the salivary gland. Med Mol Morphol. 2020. http://doi: 10.1007/s00795-020-00256-4.
- Sato K, Akiba J, Nakamura K, Abe H, Kawahara A, Aso T, et al. Mucoepidermoid carcinoma of the sublingual gland harboring a translocation of the MAML2 gene: A case report. Oncol Lett. 2017; 14: 2970-2974.
- Stevens TM, Kovalovsky AO, Velosa C, Shi Q, Dai Q, Owen RP, et al. Mammary analog secretory carcinoma, low-grade salivary duct carcinoma, and mimickers: a comparative study. Mod Pathol. 2015; 28: 1084-1100.
- Seifert G, Donath K. Multiple tumours of the salivary glands--terminology and nomenclature. Eur J Cancer B Oral Oncol. 1996; 32B: 3-7.